
Elevation of serum proprotein convertase subtilisin/kexin type 9 (PCSK9) concentrations and its possible atherogenic role in patients with systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterized by various clinical manifestations and serologic findings. SLE patients have a tendency of accelerated atherosclerosis (AS) affecting life quality and mortality vitally. This phenomenon can only partly be explained by traditional risk factors of cardiovascular disease (CVD), such as atherogenic lipid profiles. Imbalanced inflammation also plays vital roles in CVD risk of SLE patients (1). Previous studies demonstrated that traditional preventive CVD therapies like statins are incapable of bringing significant benefit in lowering CVD incidence in SLE (2,3). Therefore, there is an urgent need for increasing understanding of new mechanisms and novel target for treatment.
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a serine protease mainly synthetized in liver. The association between PCSK9 and CVD risk had been widely reported: PCSK9 enhances CVD risk by both regulating cholesterol metabolism (via reducing clearance of low-density lipoprotein) and acting as a critical regulator of atherogenic inflammation (via reducing elimination of pathogenic lipids and linking with Toll like receptors/NF-kB pathway) (4-6). Accordingly, due to dual mechanisms in lipids and inflammation, PCSK9 inhibitors had proved to be highly promising drugs bringing added cardiovascular benefit in ODYSSEY LONG TERM trial and FOURIER trial (7-9).
To our knowledge, no evidence had established the link between PCSK9 and accelerated AS in SLE. Moreover, clinical analysis of SLE patients and age/sex-matched controls revealed that accelerated AS is mainly confined to a subgroup of patients with lupus nephritis (LN) (10). In this study, we aimed to assess serum PCSK9 levels and CVD risk factors between: (I) SLE patients and controls; (II) SLE patients with and without evidence of AS [defined as carotid intima-media thickness (cIMT) >1.0 mm]; (III) SLE patients with and without LN. Univariate and multivariate linear regression analysis were applied in SLE patients to investigate the correlation among PCSK9 concentrations, SLE disease parameters and atherogenic risks factors. Considering hydroxychloroquine (HCQ) was described as the cornerstone drug in SLE and having protective effects against accelerated AS in SLE patients targeting toll-like receptor signaling and subsequent inflammation (11), effects on PCSK9 concentrations and inflammatory biomarker like C-reactive protein (CRP) by monotherapy with HCQ were also analyzed in our study.
Methods
Patients and controls
One hundred and forty individuals including 90 patients with SLE and 50 age- and sex-matched controls were consecutively recruited at Second Affiliated Hospital of Fujian Medical University from September 2016 and February 2017. SLE patients fulfilled the 2009 American College of Rheumatology (ACR)/Systemic Lupus International Collaborating Clinics (SLICC) criteria for SLE (12). Volunteer blood donors and hospital staff served as healthy controls. Negativity of antinuclear antibodies was essential for healthy controls included. Individuals with history of symptomatic CVD, obesity, stroke, glomerular filtration rate <60 mL/(min·1.73 m2), hypertension, smoking, diabetes, gout, current infections, tumor or statin use were excluded. Informed consent was obtained from all individuals included. Data of disease activity assessed by systemic lupus erythematosus disease activity index (SLEDAI) was recorded. cIMT was measured in SLE patients and healthy controls. According to the measured value of cIMT, SLE patients were divided into SLE-AS subgroup and SLE-NonAS subgroup (cut-off point: 1.0 mm). The study was carried out in accordance with the Declaration of Helsinki. The study was approved by the Research Ethics Committee of the Hospital (approval No. 2016-51).
Laboratory analysis
Peripheral blood samples for quantification of serum PCSK9 levels were centrifuged. Serums of all individuals were separated and stored at −80 °C until use. PCSK9 concentrations were quantified using sandwich enzyme-linked immunosorbent assays according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA). Serum levels of total cholesterol (TCHO), LDL cholesterol (LDL-C), HDL cholesterol (HDL-C), triglycerides (TG), fasting blood glucose (FBG), uric acid (UA), inflammatory reactants such as CRP and erythrocyte sedimentation rate (ESR) were measured using standard methods.
Statistical analysis
Differences between patients and controls and correlations were analyzed using t-test and Pearson’s correlation analysis, respectively, if data is normally distributed. If not, nonparametric Mann-Whitney rank sum test and Spearman’s correlation analysis were applied. Univariate and multivariate linear regression analysis were used to search for disease parameter possibly responsible for PCSK9 levels variation in SLE patients. Effects on PCSK9 concentrations by three-month monotherapy with HCQ were investigated by follow-up analysis in 15 SLE patients via paired t-test. Statistical significance was defined as P<0.05. All statistical analyses were performed with GraphPad Prism software 5.0 and SPSS software 21.0.
Results
Demographic features of SLE patients and healthy controls
The characteristics of patients and controls were listed in Table 1. The median age of SLE patients and controls were 33 and 32 years old. There’s no difference of sex ratio, age, BMI, lipids parameters (TCHO, LDL-C, TG, HDL-C), FBG or UA levels between SLE patients and controls. Nevertheless, SLE patients still had significantly higher ratio of cIMT thickening than controls (30.00% versus 14.00%, P=0.03). All of SLE patients were under therapy with HCQ. Fifteen patients had withdrawn steroid use. Partial patients were under therapy with immunosuppressive agents (azathioprine, cyclosporin A or mycophenolate mofetil) (Table 1).
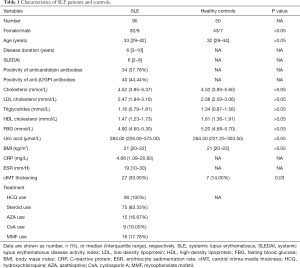
Full table
Serum PCSK9 concentrations in SLE patients
Even having comparable lipids profiles with controls, SLE patients had significantly elevated serum PCSK9 concentrations than controls (median of PCSK9 levels: 396.91 versus 264.09 ng/mL, P<0.001). Moreover, patients in SLE-AS subgroup had even higher PCSK9 concentrations than those in SLE-NonAS subgroup (median of PCSK9 levels: 559.11 versus 363.29 ng/mL, P<0.001) (Figure 1).
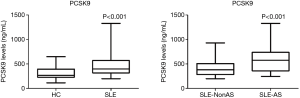
CVD risk factors between SLE-AS subgroup and SLE-NonAS subgroup
No difference of other traditional CVD risk factors (TG, TCHO, LDL-C, BMI and UA) (P>0.05) but age existed between SLE-AS subgroup and SLE-NonAS subgroup (median of age: 44 versus 32 years old, P<0.001). Patients in SLE-AS subgroup had significantly higher inflammatory biomarker (CRP and ESR) levels than those in SLE-NonAS subgroup (median of CRP: 15.73 versus 3.59 mg/L, P<0.01; median of ESR: 26 versus 17 mm/H, P<0.01) (Figure 2).

AS, PCSK9 levels, CRP levels and ESR in patients with and without LN
As the result of previous study, SLE patients with LN in our cohort had significantly higher ratio of cIMT thickening than those without LN (41.67% versus 16.67%, P<0.01). In SLE-AS subgroup, the ratios of SLE patients with LN and without LN were 74.07% and 25.93%, respectively. Furthermore, LN patients had higher PCSK9 levels, CRP levels and ESR, when compared with those without LN (median of PCSK9 levels: 529.89 versus 330.09 ng/mL, P<0.001; median of CRP levels: 9.24 versus 1.38 mg/L, P<0.001; median of ESR: 21.50 versus 14.50 mg/L, P<0.05) (Figure 3).

Cross comparing of CVD risk factors in SLE with or without LN and SLE with or without AS
In the cross comparing of CVD risk factors, SLE patients were divided into four subgroups: SLE-AS NonLN, SLE-AS LN, SLE-NonAS LN and SLE-NonAS NonLN. We found that: (I) for SLE-AS patients, those in SLE-AS LN subgroup had significantly higher PCSK9 levels, but not CRP levels or age than patients in SLE-AS NonLN subgroup (P<0.05); (II) among SLE-NonAS patients, those in SLE-NonAS LN subgroup had significantly higher CRP and PCSK9 levels, but not age than patients in SLE-NonAS NonLN subgroup (P<0.01, P<0.001, respectively); (III) among SLE-LN patients, age, but not CRP or PCSK9 levels, was significantly higher in SLE-AS LN subgroup than that in SLE-NonAS LN subgroup (P<0.01); (IV) in SLE-NonLN patients, those in SLE-AS NonLN subgroup had significantly higher age and PCSK9 levels, but not CRP levels than patients in SLE-NonAS NonLN subgroup (P<0.001, P<0.05, respectively); (V) no significant difference of BMI, TG, UA, TCHO or LDL-C was found in the cross comparing referred above.
Correlational analysis, univariate and multivariate linear regression analysis of PCSK9 levels and disease parameter in SLE patients
In the correlational analysis, PCSK9 concentrations correlated with CRP levels, age and ESR (rs=0.462, P<0.001; rs=0.236, P<0.05; rs=0.229, P<0.05, respectively), but not SLEDAI, lipids parameters (TCHO, LDL-C, ApoA1, ApoB, TG, HDL-C), BMI or UA levels. When the female SLE patients were separately analyzed, only CRP levels and age showed the correlation with statistical significance (rs=0.464, P<0.001; rs=0.223, P<0.05) (Table 2) (Figure 4).
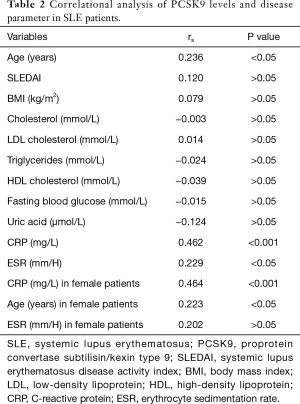
Full table
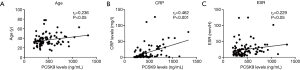
Univariate linear regression showed that CRP and age, but not ESR were significant positive predictors of PCSK9 (CRP: β 0.457, P<0.001; age: β 0.215, P=0.042; ESR: β 0.188, P>0.05). Interestingly, in multivariate linear regression using CRP, age and ESR as independent variable, only CRP, but not age or ESR, was significant positive predictors of PCSK9 (CRP: β 0.408, P<0.001; age: β 0.159, P=0.101) (Table 3).
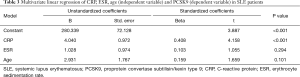
Full table
Effects on PCSK9 and CRP levels by HCQ in SLE patients
We investigated effects on PCSK9 levels by monotherapy with HCQ for three months in fifteen SLE patients with inactive disease (SLEDAI =2). Five patients had thickening of cIMT. Paired t-test showed that monotherapy with HCQ for three months significantly reduced PCSK9 levels and CRP levels in these patients (mean PCSK9 levels before and after HCQ therapy: 512.83 versus 466.10 ng/mL, P<0.01; mean CRP levels before and after HCQ therapy: 14.17 versus 9.47 mg/L, P<0.01).
Discussion
Accelerated AS in SLE is thought to be driven by the complex interplay among autoimmunity, inflammation, vascular repair, traditional risk factors and therapeutic agents (1). In this study, first of all, no significant difference of age, sex ratio, lipids parameters, FBG, BMI or UA levels existed between SLE patients and controls. However, SLE patients still had significantly higher ratio of cIMT thickening than controls. Similarly, in the subgroup analysis of atherogenic factors between SLE-AS and SLE-NonAS patients, only the difference of age, but not other traditional cardiovascular risk factors had statistical significance. Interestingly, we found that patients in SLE-AS subgroup had significantly higher inflammatory biomarker (CRP and ESR) levels than those in SLE-NonAS subgroup. Similar result was obtained in the comparison between LN patients who was verified to possess higher CVD risk in our study and those SLE patients without LN. In the cross comparing of CVD risk factors in different subgroups of SLE patients, no significant difference of BMI, TG, UA, TCHO or LDL-C was found yet. Given our exclusion criteria, the findings above were conformed to the viewpoint that traditional CVD risk factors cannot completely explain AS in SLE, and inflammation also plays an important role in the pathogenesis.
Moreover, to our knowledge, we firstly reported the elevation of serum PCSK9 levels in SLE patients than controls. The relation between PCSK9 and AS had been well documented in previous studies. Dyslipidaemia and inflammation, which affect PCSK9 concentration, were also the key factors in the pathogenesis of AS. SLE patients were further divided into SLE-AS and SLE-NonAS subgroup according to the measured value of cIMT for its capability as a biomarker of AS (13). We found that patients in SLE-AS subgroup had even higher PCSK9 concentrations than those in SLE-NonAS subgroup.
Given the similar lipids profiles between: (I) SLE patients and controls; (II) SLE-AS subgroup and SLE-NonAS subgroup, we turn to inflammatory biomarkers to find out the factors linked with PCSK9 concentration variation in SLE patients. In the correlational analysis with disease parameters, PCSK9 concentrations correlated with CRP levels, age and ESR. Univariate linear regression showed that CRP and age, but not ESR, were significant positive predictors of PCSK9. Interestingly, in the further multivariate linear regression, only CRP was significant positive predictors of PCSK9. Because role of CRP in the CVD risk of SLE patients had been demonstrated in previous study (14), our result suggested the connection between PCSK9 and atherogenic inflammation in SLE. The pathway involved in the PCSK9-mediated inflammation includes: (I) Intracellular PCSK9 could exerts cytoplasmic effects that might regulate the expression of genes controlling inflammation in macrophage; (II) PCSK9 could also target LRP-1 which is involved in the activation of JAK/STAT and ERK pathways (15). Conversely, Ruscica et al. reported that inflammatory conditions induce the PCSK9 expression by the pathway depending on suppressor of cytokine signaling 3 (SOCS-3) (16). Furthermore, PCSK9 gene interference suppresses atherogenic inflammation via inhibiting the TLR4/NF-kB signaling pathway without affecting plasma cholesterol level in high-fat diet-fed apoE KO mice (6). Bernelot Moens et al. reported that PCSK9 monoclonal antibodies can reverse the pro-inflammatory profile of monocytes in familial hypercholesterolaemia (17). These findings demonstrated the positive feedback between PCSK9 and inflammation, probably independent of atherogenic lipids.
Notably, no correlation between PCSK9 levels and lipids parameters was found in our study, suggestive of that elevated PCSK9 levels in SLE patients might not be linked with variation of LDL-C levels. This phenomenon can be explained by the previous study showing that even in general population, variations in plasma PCSK9 concentrations can also only explain 7% of the variability in LDL-C (18).
As mentioned above, recent study by Gustafsson et al. revealed that accelerated AS is mainly confined to a subgroup of patients with LN (10,19). In our study, we found LN patients had higher PCSK9 concentrations than those without LN evidence, accompanied by higher incidence of cIMT thickening. The mechanisms underpinning this phenomenon are not clear but indicate a possible benefit of PCSK9 inhibition in LN patients.
Despite the lack of randomized controlled trials, available evidence strongly suggests that HCQ can bring protective effects on AS and CVD in SLE patients due to its broad spectrum of beneficial effects on inflammation and traditional CVD risk factors (11). Thus, we tested the effect of PCSK9 concentration and CRP levels before and after HCQ treatment for three months. We observed the substantial reduction of both PCSK9 and CRP levels in these patients. This finding provided indirect evidence for the possible role of PCSK9 in SLE-AS and the relation between PCSK9 and CRP.
In conclusion, our study provided first evidence that proves the elevation of serum PCSK9 concentrations in SLE patients, especially in those with AS or LN. In our cohort, PCSK9 is associated with atherogenic inflammation in SLE patients. We have also shown that HCQ, which is thought having protective effects against AS in SLE, can effectively reduce serum PCSK9 levels and inflammatory biomarker in SLE patients. These findings may be helpful to better understand the pathogenesis of AS in SLE. It is worthy of in-depth investigation in SLE-AS animal model further.
Acknowledgements
The authors thank Qiulan Li and Hongzhi Gao for the excellent technical assistance.
Funding: This work was supported by Key Clinical Specialty Discipline Construction Program of Fujian, China, Science and Technology Program of Quanzhou (grant No. 2018N014S), the Construct Foundation of the Key Clinical Discipline of Fujian Medical University (grant No. XKPY201102) and the High-level Talent Development Foundation of Fujian Province (grant No. 2109901).
Footnote
Conflicts of Interest: This manuscript has not been submitted or published elsewhere with the exception of abstracts published with scientific meetings.
Ethical Statement: The study was carried out in accordance with the Declaration of Helsinki. The study was approved by the Research Ethics Committee of the Hospital (approval No. 2016-51). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Frieri M, Stampfl H. Systemic lupus erythematosus and atherosclerosis: Review of the literature. Autoimmun Rev 2016;15:16-21. [Crossref] [PubMed]
- Schanberg LE, Sandborg C, Barnhart HX, et al. Use of atorvastatin in systemic lupus erythematosus in children and adolescents. Arthritis Rheum 2012;64:285-96. [Crossref] [PubMed]
- van Leuven SI, Mendez-Fernandez YV, Wilhelm AJ, et al. Mycophenolate mofetil but not atorvastatin attenuates atherosclerosis in lupus-prone LDLr(-/-) mice. Ann Rheum Dis 2012;71:408-14. [Crossref] [PubMed]
- Giugliano RP, Sabatine MS. Are PCSK9 Inhibitors the Next Breakthrough in the Cardiovascular Field? J Am Coll Cardiol 2015;65:2638-51. [Crossref] [PubMed]
- Walley KR, Thain KR, Russell JA, et al. PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci Transl Med 2014;6:258ra143. [Crossref] [PubMed]
- Tang ZH, Peng J, Ren Z, et al. New role of PCSK9 in atherosclerotic inflammation promotion involving the TLR4/NF-κB pathway. Atherosclerosis 2017;262:113-22. [Crossref] [PubMed]
- Dadu RT, Ballantyne CM. Lipid lowering with PCSK9 inhibitors. Nat Rev Cardiol 2014;11:563-75. [Crossref] [PubMed]
- Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1489-99. [Crossref] [PubMed]
- Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med 2017;376:1713-22. [Crossref] [PubMed]
- Gustafsson JT, Herlitz Lindberg M, Gunnarsson I, et al. Excess atherosclerosis in systemic lupus erythematosus,-A matter of renal involvement: Case control study of 281 SLE patients and 281 individually matched population controls. PLoS One 2017;12:e0174572. [Crossref] [PubMed]
- Floris A, Piga M, Mangoni AA, et al. Protective Effects of Hydroxychloroquine against Accelerated Atherosclerosis in Systemic Lupus Erythematosus. Mediators Inflamm 2018;2018:3424136. [Crossref] [PubMed]
- Petri M. SLICC revision of the ACR classification criteria for SLE. Arthritis Rheum 2009;60:895.
- Belibou C, Ancuţa C, Ancuţa E, et al. Carotid intima-media thickness and plaque as surrogate biomarkers of atherosclerosis among consecutive women with systemic lupus erythematosus. Rom J Morphol Embryol 2012;53:29-34. [PubMed]
- Dima A, Opris D, Jurcut C, et al. Is there still a place for erythrocyte sedimentation rate and C-reactive protein in systemic lupus erythematosus? Lupus 2016;25:1173-9. [Crossref] [PubMed]
- Norata GD, Tavori H, Pirillo A, et al. Biology of proprotein convertase subtilisin kexin 9: beyond low-density lipoprotein cholesterol lowering. Cardiovasc Res 2016;112:429-42. [Crossref] [PubMed]
- Ruscica M, Ricci C, Macchi C, et al. Suppressor of Cytokine Signaling-3 (SOCS-3) Induces Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Expression in Hepatic HepG2 Cell Line. J Biol Chem 2016;291:3508-19. [Crossref] [PubMed]
- Bernelot Moens SJ, Neele AE, Kroon J, et al. PCSK9 monoclonal antibodies reverse the pro-inflammatory profile of monocytes in familial hypercholesterolaemia. Eur Heart J 2017;38:1584-93. [Crossref] [PubMed]
- Lakoski SG, Lagace TA, Cohen JC, et al. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab 2009;94:2537-43. [Crossref] [PubMed]
- McHugh J. Systemic lupus erythematosus: Atherosclerosis confined to patients with nephritis. Nat Rev Rheumatol 2017;13:322. [PubMed]


