Interpretation and clinical practice of regulation for prevention and control of healthcare associated infection in outpatient and emergency department in healthcare facilities
Introduction
In China, huge number of outpatient and emergency patients and their family members gather in outpatient and emergency department (OED). It is reported that the total number of hospital visits was 3.44 billion (42.1% of the total number of visits) in 2017 (1), which easily result in the spread of infectious diseases even the occurrence of outbreaks. It is more difficult for medical staff to implement infection prevention and control measures in OED, compared to inpatient care settings; and outpatient settings have traditionally lacked infrastructure and resources to support infection control and prevention activities. At the end of 2016, more than 2,000 medical institutions in China carried out day surgery (2). Ya-Hui Jiao who is the deputy director of the Bureau of Medical Administration of National Health Commission of the People’s Republic of China, said that more than half of the tertiary hospitals have undergone day surgery, and the proportion of elective surgery for day surgery has increased to 12.8%. 639 healthcare facilities have set up day surgery centers (3). Many of the day surgery were carried out in OED. This new trend does pose new challenges for infection control and prevention for healthcare personnel (HCP).
Remarkably, several cases of outbreaks in hospitals are reported in OED (4-7), which strongly suggested breakdowns in basic infection prevention procedures, demonstrating the need for greater understanding and implementation of basic infection prevention (standard precaution).
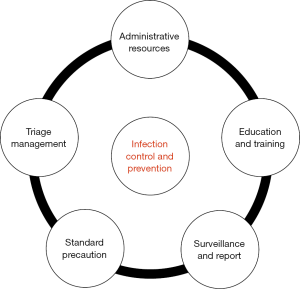
In 2009, former Ministry of Health release series guidelines for Isolation Precautions (8), Standard for nosocomial infection surveillance (9), and Hand Hygiene (10), which was a first attempt to provide recommendations that can be applied to improve the concept of standard precaution. With full consideration of the characteristics and the amount of outpatient and emergency patients in our country, this regulation was carefully established and represents the basic infection prevention expectations for safe care in OED, including Administrative resources, Education and Training, Surveillance and Reporting, Standard Precaution and Triage Management (Figure 1).
Administrative support to infection prevention
Administrative support is critical for infection control and prevention in OED. At least, it included three dimensions: (I) system building of HAIs management; (II) HCP management; (III) sufficient and appropriate equipment and supplies
System building of HAIs management
System building is a critical and long-effective mechanism in infection control and prevention of HAIs in OED. HAIs management team (HMT) and system should be built in OED of healthcare facilities. Outpatient and emergency medical staff should master and follow the relevant systems and procedures for HAIs management, especially the implementation of standard prevention measures. Infection prevention must be made a priority in any setting where healthcare is delivered. The outpatients and emergency department HMT should formulate the system, plans, measures and procedures based on the characteristics of HAIs and the actual medical work of OED, and carry out HAIs management. HMT is responsible for organizing staff to carry out training on knowledge and skills for HAIs management. OED should assure that at least one individual trained for infection control and prevention is employed or regularly available to implement the infection control and prevention program.
HCP management
This individual should be involved in the development of written infection prevention policies and have regular communication with HCP to address specific issues or concerns related to infection prevention. The development and ongoing refinement of infection prevention policies and procedures should be based on evidence-based guidelines, regulations, or standards. These policies and procedures should be tailored to the facility and re-assessed regularly (e.g., annually), taking into consideration the types of services provided by the facility and the patient population who is served. Administrators should also assure that facility policies and procedures address occupational health needs including vaccination of HCP, management of exposures or infections in personnel requiring post-exposure prophylaxis and/or work restrictions, and compliance with the relative standards and regulations.
Sufficient and appropriate equipment and supplies
This includes the availability of sufficient and appropriate equipment and supplies necessary for the consistent observation of Standard Precautions, including hand hygiene products, injection equipment, and personal protective equipment (PPE) (e.g., gloves, gowns, face and eye protection).
Education and training
Education and training are critical for ensuring that infection control and prevention policies and procedures are understood and followed. Ongoing education and competency-based training of HCP are critical for ensuring that infection prevention policies and procedures are understood and followed. Target personnel should not be limited to HCP, but also should include patients, their family members and even accompanying personnel. Education on the basic principles and practices for preventing the spread of infections should be provided to all HCP. Training should include both HCP safety and patient safety, emphasizing job- or task specific needs. Training should be provided upon orientation to the facility to maintain competency and should be repeated annually or anytime when policies or procedures are updated/revised. Competencies should be documented after each training.
Various forms of education can be carried out using folding, posters, promotional videos, etc. Training needs to considering diversified objects (administrators, clinical medical staff, logistics, etc.), diversified in form (theoretical teaching, PBL teaching, app. examination system, videos and MVs, etc.) and multi-level (Hospital and departmental scope). Various forms of HAIs control and prevention activities would be conducted periodically and be continued to innovate, such as infection prevention and control week, signature commitment wall and so on. All of these activities were created to be a culture of infection prevention and control.
Surveillance and report healthcare-associated infections
Surveillance was always applied to tracking of outcome and adherence to specific process measures as a critical means to detection of HAIs risk points.
However, HAIs surveillance in OED often encounters some challenges because patients may have higher mobility and be brief or sporadic and evaluation. At a minimum, outpatient facilities need to adhere to local, provincial and national requirements regarding reportable disease and outbreak reporting. According to the requirements of WS/T 312 (9), combined with the actual situation of hospital, comprehensive surveillance and target monitoring of health care-related infection cases, such as catheter-related bloodstream infection and surgical site infection, can be designed and implemented.
It is advisable to carry out regular monitoring of hand hygiene compliance, at least once a quarter. The monitoring method for hand hygiene compliance is recommended to be carried out in accordance with the World Health Organization Hand Health Technical Reference Manual. Regular focused practice surveys or audits (e.g., audits of infection prevention practices including hand hygiene, medication handling, reprocessing of reusable devices) offer a means to ensure ongoing compliance of HCP with recommended practice.
Adhere to standard precautions
Standard Precautions are the minimum infection prevention practices that apply to all patient care which must be strictly adhered (8) in clinical practice, regardless of suspected or confirmed infection status of the patient in any healthcare facilities. These practices are designed to both protect HCP and patients from spreading infections among patients. Standard precautions include: (I) hand hygiene; (II) use of PPE; (III) respiratory hygiene/cough etiquette; (IV) safe injection practices (including medical waste); (V) safe handling of potentially contaminated equipment; (VI) safe handling of patients’ environment. Each of these elements of Standard Precautions is described in the sections that follow.
Hand hygiene
Hand hygiene practices are crucial to reduce healthcare associated infections (HAIs), a priority for patient safety (11). Indeed, hands are the most common vehicle in the transmission of contact-borne pathogen. Insufficient hand hygiene would lead to healthcare-associated infections (10,12). Therefore, hand hygiene is also critical to interrupt the transmission risk of spreading infections in outpatient or emergency settings. Hand hygiene clinical practices should be based on different indications. Conventionally, alcohol-based hand rub (ABHR) is recommended by the National Health Commission of the People’s Republic of China (NHCPRC), CDC and the World Health Organization (WHO) because of its quick and high activity against a broad spectrum, great compliance, and irritating hands less which was wildly used in healthcare settings (13). However, hands should be washed with soap and water when hands are visibly soiled (e.g., blood, body fluids) and is also preferred after caring for a patient with known or suspected Clostridium difficile or norovirus instead of ABHR.
PPE
PPE is applied to protect HCP from exposure to or contact with suspected or confirmed infectious agents. Common PPE included gloves, gowns, face masks, respirators, goggles and face shields (8). Correct use of PPE in clinical practice is principally important. The selection of PPE is based on potential exposure to blood, body fluids or infectious agents. Hand hygiene is always the final step after removing and disposing of PPE. Each outpatient or emergency facility should evaluate potential exposure of each service they provide and apply specific sufficient and appropriate PPE. Proper selection and use of PPE at the clinical practice should be educated and trained for all HCP and make sure everyone good mastery.
Respiratory hygiene/cough etiquette
Respiratory hygiene/cough etiquette highlights the need for vigilance and prompt implementation of infection prevention measures to prevent the transmission of all respiratory infections in healthcare settings at the first point of encounter with the facility (e.g., reception and triage areas in emergency departments and outpatient clinics). They should be incorporated into infection control practices as one component of Standard Precautions. The strategy proposed has been termed Respiratory Hygiene/Cough Etiquette (14,15) and is intended to be incorporated into infection control practices as a new component of Standard Precautions. The strategy is targeted at patients and accompanying family members and friends with undiagnosed transmissible respiratory infections, and applies to any person with signs of illness including cough, congestion, rhinorrhea, or increased production of respiratory secretions when entering a healthcare facility. Additional information related to respiratory hygiene/cough etiquette can be found in the 2009 Guideline for Isolation Precautions (8).
Safe injection practices
It was reported that there were 51 outbreaks involving the notification of more than 75,000 potentially exposed patients and identification of 620 who became infected with HBV or HCV from 1998 to 2009, a majority of which occurred in emergency departments (16,17). The majority resulted from unsafe injection practices and lapses could be accounted for low adherence to standard precaution, including failure to maintain aseptic technique when preparing or administering parenteral medications (e.g., overt syringe reuse or contamination of shared parenteral medications by reused syringes); contamination of equipment and supplies (e.g., preparing medications in contaminated area); or improper use and handling of blood glucose monitoring equipment (e.g., single patient use fingerstick devices used on multiple patients or blood glucose meters not cleaned between patients) (17). To avoid complex compounding, clinics should work with pharmacy services (18). In all reported events of outbreaks, implementation of basic infection prevention measures decreased subsequent transmission (18). Injection safety includes practices intended to prevent transmission of infectious diseases between one patient and another, or between a patient and healthcare provider during preparation and administration of parenteral medications. Implementation of the Blood-borne Pathogens Standard has helped increase the protection of HCP from blood exposure and sharps injuries. For example, efforts to increase uptake of hepatitis B vaccination and implementation of safety devices that are designed to decrease risks of sharps injury are needed (19).
Safe management of medical items
OED must establish sound system and standard operation procedures (SOP) for reprocessing of reusable devices. All reusable medical devices must be cleaned and maintained according to the information for manufacturer (IFM) and the relative regulations of health administration to prevent cross-transmission of infectious agents. The Classic Spaulding Classification has been applied to determine the level of disinfection or sterilization required for reusable medical devices/equipment, including critical items that must be sterile prior to use; semi-critical items which require high-level disinfection prior to reuse; noncritical items are those that should undergo low- or intermediate-level disinfection depending on the nature and degree of contamination.
Some reprocessing procedures or SOP, such as the recycling of foreign instruments, the whole process of the cleaning, disinfection and sterilization process must be posted in a conspicuous position. Medical staff must clearly understand their mission and responsibilities for reusable devices.
Detailed recommendations for the cleaning, disinfection, and sterilization of medical devices are available in the Regulation of Disinfection Technique in Healthcare Settings (20) and Hygienic Standard for Disinfection in Hospital (21).
Medical items should be clearly labeled by the manufacturer as either reusable or single-use and should be strictly accompanied by IFM for cleaning and disinfection or sterilization. However, disposable medical supplies should be disposed of in accordance with medical waste in a timely manner and should not be reprocessed according to the Measures for the Supervision and Administration of Medical Device Use Quality of SFDA Guidance for Industry and SFDA Staff (22).
Safe handling of patients environment
OED should establish policies and procedures for routine cleaning and disinfection of environmental surfaces as part of their infection prevention plan. The facilities should provide the appropriate and enough products for cleaning and disinfecting the environmental surfaces.
The OED environment can be divided into the following three areas according to the level of being contaminated: low-risk areas, including emergency offices, emergency pharmacies, and interiors; moderate-risk areas, including emergency department halls, registration and payment windows, waiting areas, general clinics, electrocardiogram rooms, ultrasound departments and other functional examination rooms; high-risk areas, including blood collection room, dressing room, puncture room, injection room, otolaryngology clinic, gynecological clinic, infectious disease clinic, intestinal clinic, fever clinic, emergency operation room, dental clinic, hemodialysis department, endoscope department and other areas.
Trained HCP were responsible for routine cleaning and disinfection of environmental surfaces. The HCP should wear appropriate PPE before starting cleaning and disinfection. Environmental cleaning and disinfection compliance should be monitored and be assessed which was detailed described in WS/T 512 (23) and GB15982 (21).
Pre-detection and triage management
OED should establish policies and procedures for pre-detection and triage management according to the regulation of “Law of the People’s Republic of China on the Prevention and Control of Infectious Diseases” (24). Healthcare facilities should detect and disperse out-patients, and examine and distinguish infectious disease patients.
Specific syndromes involving diagnostic uncertainty (e.g., diarrhea, febrile respiratory illness, and febrile rash) are routinely encountered in OED that deserve appropriate triage. System for early detection and management of potentially infectious patients should be developed and implemented at the pre-detection and triage point of entry the facility.
Pre-detection and triage point should be equipped with thermometers, hand hygiene facilities and supplies, PPE and disinfection products, etc. so as to be used whenever needed. Standard precaution should be trained and strictly implemented for HCP at the triage point. Healthcare facilities should poster clear signs, notices, direction for guiding suspected patients.
Transmission-based precautions
Transmission-based precautions are empirical infection control and prevention measure to interrupt the potential or suspected infectious disease according to the transmitted characters of pathogens (contact precautions, droplet precautions and airborne precautions). Detailed information related to transmission-based precautions can be found in the 2009 Guideline for Isolation Precautions (8).
Conclusions
The recommendations described the basic or the minimal infection prevention measures for safe care in OED. Based on the recommendations of this regulation, healthcare facilities are encouraged to refer and further augment their own system and implement in clinical practice. For the control and prevention of HAI in the OED, it is necessary to perform continuous and dynamic monitoring, and firmly grasp the four key points: (I) Key pathogens [e.g., Carbapenem-resistant Enterobacteriaceae (CRE), Carbapenem-resistant Acinetobacter baumannii (CRAB), Carbapenem-resistant Pseudomonas aeruginosa (CRPsA), Methicillin-resistant Staphylococcus aureus (MRSA)]; (II) key facilities (e.g., fresh air systems, centralized water supply systems). If a negative pressure ward or a negative pressure operating room is set, physical parameters and microbial parameters need to be monitored according to the corresponding standards; (III) key populations (e.g., susceptible patients); (IV) key departments (e.g., Hemodialysis center, Operating room, Department of Stomatology, Central Sterile Supply Department, Endoscopy Center). Continuous and dynamic monitor, timely feedback and continuous quality improvement is extremely critical to ensure the patient safety in OED.
Acknowledgements
The authors are particularly grateful to Prof. Zhi-Yong Zong, West China Hospital of Sichuan University, who is the first draftsman of “Regulation for prevention and control of HAI in outpatient department and emergency department in healthcare facilities” (WS/T 591-2018), for his kind instruction.
Funding: The present study was supported by grants from the National key Research & Development plan of Ministry of Science and Technology of the People’s Republic of China (Grant no. 2018YFC1314900, 2018YFC1314901), the 2016 industry prospecting and common key technology key projects of Jiangsu Province Science and Technology Department (Grant no. BE2016002-4), the 2017 projects of Jiangsu Provincial Department of Finance (Grant no. 2150510), the 2016 projects of Nanjing Science Bureau (Grant no. 201608003) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) (JX10231801).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- National Health Commission of the People’s Republic of China. Statistical bulletin of health and health development in China in 2017. Available online: http://www.nhfpc.gov.cn/guihuaxxs/s10743/201806/44e3cdfe11fa4c7f928c879d435b6a18.shtml
- People’s Daily. More than 2000 medical institutions in China carry out daytime surgery. Available online: http://health.ce.cn/news/201706/09/t20170609_5321299.shtml
- China Hospital CEO. Ya-Hui Jiao: More than half of the tertiary hospitals carry out day surgery. Available online: http://www.h-ceo.com/zixun/shizheng/2017-12-08/1478.html
- See I, Bagchi S, Booth S, et al. Outbreak of Clostridium difficile Infections at an Outpatient Hemodialysis Facility—Michigan, 2012–2013. Infect Control Hosp Epidemiol 2015;36:972-4. [Crossref] [PubMed]
- Yuen KY, Seto WH, Ching TY, et al. An outbreak of Candida tropicalis peritonitis in patients on intermittent peritoneal dialysis. J Hosp Infect 1992;22:65-72. [Crossref] [PubMed]
- Ibrahim LA, Sellick JA, Watson EL, et al. An Outbreak of Severe Group A Streptococcus Infections Associated with Podiatric Application of a Biologic Dermal Substitute. Infect Control Hosp Epidemiol 2016;37:306-12. [Crossref] [PubMed]
- Epstein L, Hunter JC, Arwady MA, et al. New Delhi metallo-β-lactamase-producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA 2014;312:1447-55. [Crossref] [PubMed]
- Technique standard for isolation in hospitals: WS/T 311-2009 [S/OL]. [2009-04-01]. Available online: http://www.moh.gov.cn/zhuz/s9496/200904/40116.shtml
- Standard for nosocomial infection surveillance: WS/T 312-2009. Available online: http://www.moh.gov.cn/zhuz/s9496/200904/40117.shtml
- Standard for hand hygiene for healthcare workers in healthcare settings: WS/T 313-2009. Available online: http://www.moh.gov.cn/zhuz/s9496/200904/40118.shtml.
- Pittet D, Donaldson L. Clean Care is Safer Care: a worldwide priority. Lancet 2005;366:1246-7. [Crossref] [PubMed]
- Masson-Roy S, Saito H, Pittet D. The WHO 2018 Hand Hygiene Campaign: Make a Difference-Prevent Sepsis in Health Care. Am J Respir Crit Care Med 2018;197:985-6. [Crossref] [PubMed]
- Longtin Y, Sax H, Allegranzi B, et al. Videos in clinical medicine. Hand hygiene. N Engl J Med 2011;364:e24. [Crossref] [PubMed]
- Srinivasan A, McDonald LC, Jernigan D, et al. Foundations of the severe acute respiratory syndrome preparedness and response plan for healthcare facilities. Infect Control Hosp Epidemiol 2004;25:1020-5. [Crossref] [PubMed]
- Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD). Respiratory Hygiene/Cough Etiquette in Healthcare Settings. Available online: https://www.cdc.gov/flu/professionals/infectioncontrol/resphygiene.htm
- Thompson ND, Perz JF, Moorman AC, et al. Nonhospital health care-associated hepatitis B and C virus transmission: United States, 1998-2008. Ann Intern Med 2009;150:33-9. [Crossref] [PubMed]
- Pugliese G, Gosnell C, Bartley JM, et al. Injection practices among clinicians in United States health care settings. Am J Infect Control 2010;38:789-98. [Crossref] [PubMed]
- Ross K, Mehr J, Carothers B, et al. Outbreak of septic arthritis associated with intra-articular injections at an outpatient practice - New Jersey, 2017. MMWR Morb Mortal Wkly Rep 2017;66:777-9. [Crossref] [PubMed]
- Guideline for prevention and control for occupational exposure to bloodborne pathogen: GBZ/T 213-2008. Available online: http://www.nhc.gov.cn/zhuz/pyl/200909/42930.shtml
- Regulation of Disinfection Technique in Healthcare Settings: WS/T 367-2012. Available online: http://www.sific.com.cn/static/upload/source/20170113/25458789b438aa9c1.pdf
- Hygienic Standard for Disinfection in Hospitals: GB15982-2012. Available online: http://www.nhc.gov.cn/zhuz/s9488/201410/0e39d3b287e347ccb317a16ae2a4899f.shtml
- China Food and Drug Administration. Measures for the Supervision and Administration of Medical Device Use Quality. Available online: http://samr.cfda.gov.cn/WS01/CL0053/132880.html
- Regulation for cleaning and disinfection management of environmental surface in healthcare: WS/T 512-2016. Available online: http://www.moh.gov.cn/zhuz/s9496/201701/0a2cf2f4e7d749aa920a907a56ed6890.shtml
- National Health Commission of the People’s Republic of China. Law of the People's Republic of China on the Prevention and Control of Infectious Diseases. Available online: http://www.moh.gov.cn/fzs/s3576/201808/6d00c158844f42c5bcf94993bffa665a.shtml