Diagnostic performance of C-reactive protein for parapneumonic pleural effusion: a meta-analysis
Introduction
Pneumonia is still an important and common cause of illness and mortality worldwide; Parapneumonic pleural effusion (PPE) refers to any effusion secondary to pneumonia or lung infection (1). PPEs have traditionally been classified into three categories: uncomplicated PPE, which can be easily resolved by the antibiotic therapy; complicated parapneumonic pleural effusion (CPPE), which requires invasive treatments such as chest tube placement or surgery to cure; and empyema, which must always be drained and can be classified as CPPE to some extent (2). PPE is a common complication of pneumonia, and in a study involving 4,715 consecutive patients with community-acquired pneumonia, 882 (19%) patients had radiological evidence of pleural fluid, of whom 261 (30%) met the criteria for empyema/CPPE (3). In an analysis based on more than 3,000 consecutive thoracenteses, PPE was the third leading cause of pleural effusion (4). The presence of pleural effusion may cause confusion for clinicians since the differential diagnosis of pleural effusion can be so difficult; PPE must be differentiated from other causes of pleural effusion, including malignant pleural effusion, tuberculous pleural effusion, heart failure-associated pleural effusion, etc. (5). Making accurate and rapid diagnoses of PPE and CPPE may be of great importance for the management of these patients and providing timely treatment and avoid unnecessary invasive examinations.
C-reactive proteins (CRPs), known as “acute-phase proteins,” are produced early in the inflammatory process and provide enhanced protection against microorganisms, limits tissue damage and promotes a rapid return to a homeostatic state during infection (6). CRP is increased in the serum/plasma of patients with pneumonia and plays a valuable role in the diagnosis of pneumonia (7). Many studies also confirmed that circulating CRP may “leak” into the pleural cavity, and increased pleural CRP may present a possible biomarker for pleural infection. In fact, an increasing number of studies have reported that both serum and pleural CRP can play a role in diagnosing PPE and differentiating UPPE from CPPE, although with inconclusive results (8,9). To provide a more objective and comprehensive conclusion, this study attempts to summarize the overall diagnostic performance of serum and pleural levels of CRP for PPE/CPPE through a meta-analysis based on the current available publications.
Methods
Literature search
A literature search was performed in PubMed, EMBASE, Scopus, and Web of Science by two independent authors (D Li and Y Shen). The search terms included “C-reactive protein or CRP” AND “parapneumonic pleural effusion or parapneumonic effusion or uncomplicated parapneumonic pleural effusion or uncomplicated parapneumonic effusion or complicated parapneumonic pleural effusion or complicated parapneumonic effusion or pleural infection or infectious pleural effusion” AND “Sensitivity or specificity or accuracy”. The search included published literature up to March 1, 2018. References from eligible original and review articles were manually checked to identify additional potential studies.
Study selection
The inclusion criteria were set as follows: (I) they were diagnostic studies using CRP to diagnose PPE or CPPE in humans; (II) data for sensitivity and specificity could be extracted from the individual study; (III) the study used serum or pleural effusion for assay samples; and (IV) the study was published in English. Conference abstracts or letters to the editor with limited information were excluded. Studies with a limited number of subjects (<20) were also excluded to avoid selection bias. Two reviewers (D Li and Y Shen) independently select eligible studies, and discrepancies in selection were resolved by discussion.
Data collection
The full-text articles of all eligible publications were reviewed by two independent authors (D Li and Y Shen). Any discrepancies were resolved by discussion with a third author (J Qin) to reach a final consensus. The data extracted included the following: last name of author, year of publication, country of study, sample size, details of controls, assay samples, CRP assay method, and CRP cut-off value. The true positive (TP), false positive (FP), false negative (FN), and true negative (TN) values for each study were also extracted by calculating the sensitivity and specificity.
Study quality assessment
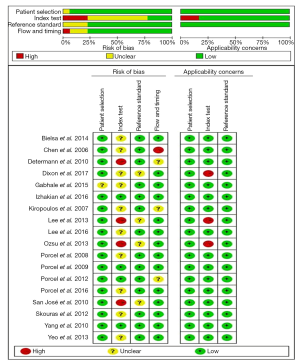
We evaluated the methodological quality of the eligible studies by using the QUADAS-2 tool (10). QUADAS-2 assesses risk of bias in 4 domains (patient selection, index test, reference standard, flow and timing) and applicability concerns in 3 domains. A result of “Yes,” “Unclear” or “No” was given according to the criterion. The responses for each criterion were then converted into risk of bias and applicability concerns as low, high, or unclear. A QUADAS plot was then created using Review Manager software (version 5.2, the Cochrane Collaboration).
Meta-analysis
The standard methods recommended for diagnostic meta-analysis and systematic review were used (11). First, we determined the diagnostic accuracy of CRP for PPE (all kinds of PPE vs. controls); then, among available patients with PPE, we calculated the ability of CRP to differentiate UPPE from CPPE (including empyema). If one study used both serum and pleural effusion samples, each sample was treated as a separate study. The following indexes of diagnostic accuracy were pooled for each study using a bivariate regression model: sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR). The diagnostic threshold identified for each study was used to plot a summary receiver operating characteristic (SROC) curve (12). The area under the SROC curve (AUC) was used to assess the overall diagnostic performance of CRP. The interstudy heterogeneity was calculated by the chi-square-based Q test and the inconsistency index I2. A significant Q test (P<0.05 or I2>50%) indicated heterogeneity among the studies. Since publication bias is of concern in diagnostic meta-analyses containing more than nine studies, we tested for bias using Deeks’ funnel plots (13). Analyses were performed using the “Midas” module in STATA, version 12.0 (Stata Corporation, College Station, TX, USA). A two-sided P value of <0.05 was considered significant.
Results
Characteristics of included studies
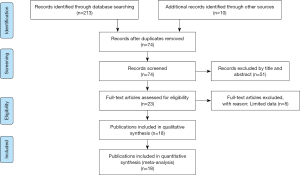
After a systematic literature search and selection, 18 publications containing 3,000 subjects (without overlap) were included in the meta-analysis (14-31). The process of study selection is shown in Figure S1. The studies were performed in nine countries and published from 2006 to 2017. Nine studies investigated the diagnostic performance of pleural CRP for PPE (15,16,18-21,24,27,28), and five studies used serum CRP to diagnose PPE (19,23,25,30,31). Seven assessed the potential of pleural CRP for differentiating CPPE from UPPE (14,16,17,21,22,26,29), two of which also determined the diagnostic role of serum CRP for CPPE (22,26).
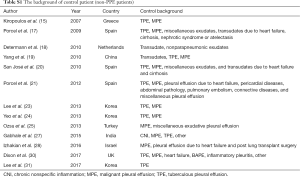
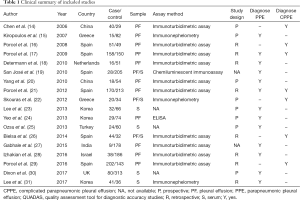
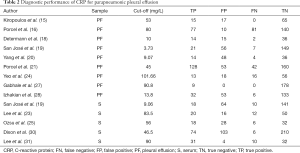
All studies supplied the definition of PPE and CPPE, with PPE referring to any pleural exudates due to bacterial pneumonia, lung abscess or bronchiectasis, while CPPE was defined as nonpurulent effusions that required an invasive procedure, such as tube thoracostomy, for effective resolution, which was widely accepted in the area of study area for PPE. For studies using CRP to diagnose PPE, the control groups included tuberculous pleural effusion, malignant pleural effusion, nonparapneumonic exudates, and transudates due to heart failure or other causes (Table S1). The CRP levels were mainly measured by immunoturbidimetric assays, immunonephelometry, chemiluminescent immunoassays, and enzyme-linked immunosorbent assays. All studies supplied the CRP cut-off value. The clinical summary for each included study is listed in Table 1. Tables 2,3 summarize the diagnostic performance of CRP for PPE and CPPE, respectively.
Full table
Full table
Full table

Full table
Quality assessment of included studies
In the included studies, a high risk of bias in the patient selection domain was mainly due to an unclear description of patient enrollment (27). The high risk of bias in the index test domain was primarily from unclear reporting regarding whether the reference standard results were known prior to interpreting the CRP and whether a threshold was prespecified (18,19,23,25). The flow and timing domains demonstrated a high risk of bias because of patients receiving different reference standards and a lack of reporting of the time between the index test and reference standard (14). These studies generally did well in the reference standard domain, with the exception of three studies providing insufficient information about the assay methods for CRP, resulting in an unclear risk of bias and high applicability concerns (23,25,30). Figure 1 shows a summary of the quality of the included studies.
Diagnostic accuracy of CRP for PPE
Summary estimates of the diagnostic performance of pleural CRP for PPE were as follows: sensitivity, 0.80 (95% CI: 0.62–0.90) (Figure 2A); specificity, 0.82 (95% CI: 0.64–0.93) (Figure 2B); PLR, 4.51 (95% CI: 1.91–10.68); NLR, 0.25 (95% CI: 0.12–0.52); and DOR, 18.26 (95% CI: 4.32–77.18). The AUC was 0.88 (95% CI: 0.84–0.90) (Figure 2C). Heterogeneity examinations suggested that the I2 values of sensitivity and specificity were 89.09% and 95.24%, respectively, with both P values <0.05, indicating significant heterogeneity among the included studies.
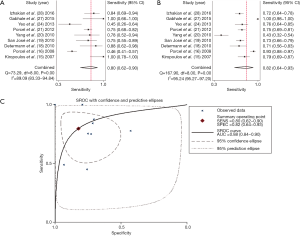
There were five studies that assessed the value of serum CRP in diagnosing PPE, and the corresponding sensitivity, specificity, PLR, NLR and DOR were 0.77 (95% CI: 0.64–0.86) (Figure 3A), 0.71 (95% CI: 0.61–0.79) (Figure 3B), 2.61 (95% CI: 1.91–3.57), 0.33 (95% CI: 0.20–0.54), and 7.96 (95% CI: 3.92–16.23), respectively. The AUC was 0.79 (95% CI: 0.75–0.83) (Figure 3C).
Diagnostic accuracy of CRP for CPPE
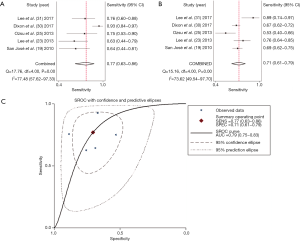
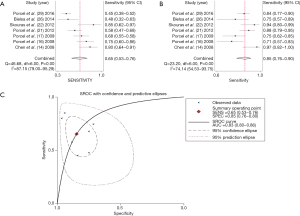
Next, we investigated the ability of CRP in differentiating CPPE from UPPE within seven studies involving a total of 942 patients with PPE. The summary estimates of the diagnostic performance of pleural CRP were as follows: sensitivity, 0.65 (95% CI: 0.53–0.76) (Figure 4A); specificity, 0.85 (95% CI: 0.76–0.90) (Figure 4B); PLR, 4.26 (95% CI: 2.49–7.29); NLR, 0.41 (95% CI: 0.29–0.59); and DOR, 10.38 (95% CI: 4.46–24.19). The AUC was 0.83 (95% CI: 0.80–0.86) (Figure 4C). Since only two studies evaluated the diagnostic potential of serum CRP for CPPE, we could not perform a meta-analysis to summarize the diagnostic performance. Table 4 summarizes the overall diagnostic performance of CRP.
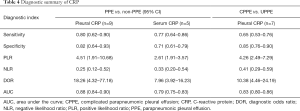
Full table
Publication bias detection
Deeks’ funnel plot asymmetry test was used to evaluate the likelihood of publication bias. Although the funnel plots for publication bias appear to suggest some asymmetry due to the limited number of studies (Figure 5), the P values associated with Deeks’ test were not significant (P=0.31), suggesting a low likelihood of publication bias among the studies evaluating the diagnostic potential of pleural CRP for PPE.
Discussion
The early identification of PPE and CPPE may benefit patients with timely treatment and avoid unnecessary tests, especially for CPPE patients who need invasive treatment (32). CRP, a classical inflammatory biomarker, has been widely used in diagnosing infectious diseases, including community-acquired pneumonia, sepsis, etc. (33). In this study, we summarized the overall performance of CRP for diagnosing PPE and further determined the accuracy of CRP in differentiating CPPE. We found that CRP shows a moderate ability for diagnosing PPE and differentiating CPPE and that CRP should be used in combination with other markers.
Nine studies with 1,704 subjects were used to evaluate the accuracy of pleural CRP in diagnosing PPE. The sensitivity and specificity of pleural CRP in diagnosing PPE were 0.80 and 0.82, respectively. Both sensitivity and specificity were moderate, which indicates that 20% of the PPE patients will have a missed diagnosis, and 18% of patients with other causes of pleural effusion will be misdiagnosed as PPE. The DOR, a single indicator of test performance in diagnostic meta-analysis (34), was 18.26, suggesting that CRP may be a helpful ancillary marker when interpreted together with additional diagnostic markers. Likelihood ratios >10 and <0.1 are considered strong indicators to rule in or rule out a diagnosis, respectively. In the present meta-analysis, the PLR was 4.51, and NLR was 0.25, suggesting a limited ability to discriminate PPE from controls. The AUC of pleural CRP was 0.88, with a medium diagnostic performance. The AUC of serum CRP was only 0.79, lower than in pleural effusion. Thus, the clinical results of pleural/serum CRP tests should be interpreted with caution.
The treatment of PPE is challenging due to the decision of whether or not to insert chest tubes, and CPPEs require semi-invasive (e.g., therapeutic thoracentesis and chest tube) or invasive (e.g., surgery) interventions for a cure, besides antibiotics (32). Many studies support a pleural pH value <7.20 or a glucose level <60 mg/dL as a treatment threshold for chest tube insertion in CPPE, but pleural pH measurements lack sufficient sensitivity and are affected by the sample collection method (32,35). Many studies have investigated the role of pleural CRP in differentiating CPPE from UPPE, and our meta-analysis included seven such studies with 942 patients with PPE. Our results found that sensitivity and specificity of CRP in differentiating CPPE from UPPE were 0.65 and 0.85, respectively, suggesting a relatively high missed diagnosis (35%) and misdiagnosis rate (15%). The AUC was 0.83, which means that pleural CRP may help distinguish CPPE from UPPE but only with a moderate discriminatory ability.
The combination of multiple markers may increase the diagnostic accuracy for PPE. In Porcel et al.’s study, the combination of pleural CRP and pleural neutrophils increased the sensitivity of diagnosing PPE from 0.75 to 0.91, which significantly increased the ability to identify PPE (21). The combination of CRP and pH increased the sensitivity of diagnosing CPPE from 0.58 to 0.79 (21). We suggest that the clinical utility of CRP should be combined with other traditional infectious markers, such as procalcitonin and triggering receptor expressed on myeloid cells-1, and biochemical markers, such as pH, pleural neutrophils and protein (18,19,21), to increase the diagnostic accuracy.
In fact, in 2012, a systematic review was published regarding the diagnostic role of procalcitonin and CRP for PPE (36). However, at the time, there were only three studies that investigated the diagnostic accuracy of CRP for PPE. In the past few years, additional studies have been published, providing more clinical evidence to support CRP as a diagnostic marker for PPE. Additionally, we separated the data of CPPE patients from all PPE patients, we summarized the role of pleural CRP in distinguishing CPPE from UPPE, and we supplied more evidence of CRP in guiding the invasive management of PPE patients. We also noticed significant differences in the cut-off values of CRP, which may be attributed to the different clinical contexts of the patient. Based on QUADAS-2 results, four studies caused a high risk of bias in the index test because there was unclear reporting regarding whether the reference standard results were known prior to interpreting the CRP values and whether a threshold was prespecified (18,19,23,25). A good diagnostic study requires the operator to be blind to the information of cases, and in independent clinical samples, a fixed threshold value may decrease the diagnostic accuracy (10), which should be clearly stated in further studies. The method of assaying CRP levels varied among the included studies, and three studies did not report the CRP assay method (23,25,30), which caused some bias for reference standard domain. Future studies should pay attention to the standard process of CRP measurement in pleural effusion.
Our meta-analysis has several limitations to address. First, although we performed an extensive systematic literature search in the main databases, only 18 publications were included in the meta-analysis after a strict selection criterion. The limited number of studies may not have been sufficient to give a definitive conclusion of whether CRP is a valuable marker for PPE or CPPE. Second, although we observed significant heterogeneity among the included studies, we did not perform a meta-regression to investigate the possible sources of heterogeneity due to the limited number of studies. Third, to guarantee the quality of the meta-analysis, we included only English articles in a limited number of databases; thus, the meta-analysis results may be biased by the omission of unpublished studies, studies published in other languages or not indexed in the databases we searched. Further studies should be well designed and performed on a large scale to validate the potential of CRP as a biomarker in diagnosing PPE.
Conclusions
In summary, CRP can play a role in diagnosing PPE and differentiating CPPE from UPPE; however, the results of CRP assays should be interpreted with other markers. More studies are needed to confirm the findings of this study.
Acknowledgements
Funding: This work was supported by a grant from the National Key Research and Development Program in China (grant number 2016YFC1304500) and the Sichuan Science and Technology Support Project (grant number 2015SZ0151). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Psallidas I, Corcoran JP, Rahman NM. Management of parapneumonic effusions and empyema. Semin Respir Crit Care Med 2014;35:715-22. [Crossref] [PubMed]
- Porcel JM. Pleural fluid tests to identify complicated parapneumonic effusions. Curr Opin Pulm Med 2010;16:357-61. [Crossref] [PubMed]
- Falguera M, Carratalà J, Bielsa S, et al. Predictive factors, microbiology and outcome of patients with parapneumonic effusion. Eur Respir J 2011;38:1173-9. [Crossref] [PubMed]
- Porcel JM, Esquerda A, Vives M, et al. Etiology of pleural effusions: analysis of more than 3,000 consecutive thoracenteses. Arch Bronconeumol 2014;50:161-5. [PubMed]
- Saguil A, Wyrick K, Hallgren J. Diagnostic approach to pleural effusion. Am Fam Physician 2014;90:99-104. [PubMed]
- Simon L, Gauvin F, Amre DK, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis 2004;39:206-17. [Crossref] [PubMed]
- Torres A, Ramirez P, Montull B, et al. Biomarkers and community-acquired pneumonia: tailoring management with biological data. Semin Respir Crit Care Med 2012;33:266-71. [Crossref] [PubMed]
- Ji Q, Huang B, Wang M, et al. Pleural fluid prealbumin and C-reactive protein in the differential diagnosis of infectious and malignant pleural effusions. Exp Ther Med 2014;7:778-84. [Crossref] [PubMed]
- Yilmaz Turay U, Yildirim Z, Türköz Y, et al. Use of pleural fluid C-reactive protein in diagnosis of pleural effusions. Respir Med 2000;94:432-5. [Crossref] [PubMed]
- Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. [Crossref] [PubMed]
- Shen Y, Zhu H, Wan C, et al. Can cholesterol be used to distinguish pleural exudates from transudates? evidence from a bivariate meta-analysis. BMC Pulm Med 2014;14:61. [Crossref] [PubMed]
- Jones CM, Athanasiou T. Summary receiver operating characteristic curve analysis techniques in the evaluation of diagnostic tests. Ann Thorac Surg 2005;79:16-20. [Crossref] [PubMed]
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882-93. [Crossref] [PubMed]
- Chen SC, Chen W, Hsu WH, et al. Role of pleural fluid C-reactive protein concentration in discriminating uncomplicated parapneumonic pleural effusions from complicated parapneumonic effusion and empyema. Lung 2006;184:141-5. [Crossref] [PubMed]
- Kiropoulos TS, Kostikas K, Oikonomidi S, et al. Acute phase markers for the differentiation of infectious and malignant pleural effusions. Respir Med 2007;101:910-8. [Crossref] [PubMed]
- Porcel JM, Galindo C, Esquerda A, et al. Pleural fluid interleukin-8 and C-reactive protein for discriminating complicated non-purulent from uncomplicated parapneumonic effusions. Respirology 2008;13:58-62. [Crossref] [PubMed]
- Porcel JM, Vives M, Cao G, et al. Biomarkers of infection for the differential diagnosis of pleural effusions. Eur Respir J 2009;34:1383-9. [Crossref] [PubMed]
- Determann RM, Achouiti AA, El Solh AA, et al. Infectious pleural effusions can be identified by sTREM-1 levels. Respir Med 2010;104:310-5. [Crossref] [PubMed]
- San José ME, Valdés L, Vizcaíno LH, et al. Procalcitonin, C-reactive protein, and cell counts in the diagnosis of parapneumonic pleural effusions. J Investig Med 2010;58:971-6. [Crossref] [PubMed]
- Yang HB, Xie KQ, Deng JM, et al. Expression of soluble Toll-like receptors in pleural effusions. Chin Med J (Engl) 2010;123:2225-30. [PubMed]
- Porcel JM, Bielsa S, Esquerda A, et al. Pleural fluid C-reactive protein contributes to the diagnosis and assessment of severity of parapneumonic effusions. Eur J Intern Med 2012;23:447-50. [Crossref] [PubMed]
- Skouras V, Boultadakis E, Nikoulis D, et al. Prognostic value of C-reactive protein in parapneumonic effusions. Respirology 2012;17:308-14. [Crossref] [PubMed]
- Lee SH, Lee EJ, Min KH, et al. Procalcitonin as a diagnostic marker in differentiating parapneumonic effusion from tuberculous pleurisy or malignant effusion. Clin Biochem 2013;46:1484-8. [Crossref] [PubMed]
- Yeo CD, Kim JW, Cho MR, et al. Pleural fluid pentraxin-3 for the differential diagnosis of pleural effusions. Tuberc Respir Dis (Seoul) 2013;75:244-9. [Crossref] [PubMed]
- Ozsu S, Abul Y, Mentese A, et al. Pentraxin-3: A novel biomarker for discriminating parapneumonic from other exudative effusions. Respirology 2013;18:657-62. [Crossref] [PubMed]
- Bielsa S, Valencia H, Ruiz-González A, et al. Serum C-reactive protein as an adjunct for identifying complicated parapneumonic effusions. Lung 2014;192:577-81. [Crossref] [PubMed]
- Gabhale S, Taparia P, Yadav D, et al. Usefulness of pleural fluid CRP level in differential diagnosis of exudative pleural effusion-A pilot study. Int J Clin Biochem Res 2015;2:97-109.
- Izhakian S, Wasser WG, Fox BD, et al. The Diagnostic Value of the Pleural Fluid C-Reactive Protein in Parapneumonic Effusions. Dis Markers 2016;2016:7539780. [Crossref] [PubMed]
- Porcel JM, Valencia H, Bielsa S. Factors influencing pleural drainage in parapneumonic effusions. Rev Clin Esp 2016;216:361-6. [Crossref] [PubMed]
- Dixon G, Lama-Lopez A, Bintcliffe OJ, et al. The role of serum procalcitonin in establishing the diagnosis and prognosis of pleural infection. Respir Res 2017;18:30. [Crossref] [PubMed]
- Lee J, Yoo SS, Lee SY, et al. Pleural fluid adenosine deaminase/serum C-reactive protein ratio for the differentiation of tuberculous and parapneumonic effusions with neutrophilic predominance and high adenosine deaminase levels. Infection 2017;45:59-65. [Crossref] [PubMed]
- Porcel JM. Distinguishing complicated from uncomplicated parapneumonic effusions. Curr Opin Pulm Med 2015;21:346-51. [Crossref] [PubMed]
- Ansar W, Ghosh S. C-reactive protein and the biology of disease. Immunol Res 2013;56:131-42. [Crossref] [PubMed]
- Glas AS, Lijmer JG, Prins MH, et al. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol 2003;56:1129-35. [Crossref] [PubMed]
- Porcel JM. Pleural fluid biomarkers: beyond the Light criteria. Clin Chest Med 2013;34:27-37. [Crossref] [PubMed]
- Zou MX, Zhou RR, Wu WJ, et al. The use of pleural fluid procalcitonin and C-reactive protein in the diagnosis of parapneumonic pleural effusions: a systemic review and meta-analysis. Am J Emerg Med 2012;30:1907-14. [Crossref] [PubMed]