
Organic acid disorders
Introduction
Inborn errors of metabolism (IEM) affecting enzymes and/or transport proteins required for catabolism of amino acids (AAs), lipids, or carbohydrates lead to pathologic buildup of upstream substrates/metabolites resulting in the clinical manifestations of organic acid disorders (OADs), also known as organic acidemias or acidurias. More than 65 specific organic acids (OAs) affecting these pathways have been identified (1). The “classical OAs” refer the most common inborn errors of branched chain AA (BCAA) catabolism, which include maple syrup urine disease (MSUD) and the isovaleric (IVA), propionic (PA), and methylmalonic (MMA) acidemias (or acidurias) (1,2).
Propiogenic essential AA (valine, methionine, isoleucine, and threonine) and odd chain fatty acids imbalance are often characteristic biochemical finding with initial clinical findings of vomiting. These both biochemical and clinical finding characteristic of classical OA ingeniously allotted a mnemonic VOMIT (valine, odd chain fatty acids, methionine, isoleucine, and threonine) was promoted by Dr. Georgianne Arnold as a common learning tool for all future Biochemical Genetics fellows in the USA (S Kanungo personal communication with IEM expert, Professors Carol Greene and Georgianne Arnold).
IEM involving lysine, its derivative hydroxylysine, tryptophan in the mitochondrial matrix leads to OA disorder with devastating neurological impairment—glutaric aciduria/acidemia type I (GA-I) and can often confused with nontraumatic brain injury or child abuse. Common childhood illnesses can lead to severe metabolic decompensation in patients with this disorder and lasting basal ganglia degeneration, even with early identification and optimal metabolic control and compliance.
Incidence of these IEM ranges from about 1 in 1,000,000 to 1 in 10,000 live births, with overall combined incidence of about 1 in 3,000 live births (1).
There is a wide range of clinical presentations, including onset and severity, however, OAs most commonly present in the neonatal period as acute metabolic collapse, though chronic progressive forms can present later in childhood as unexplained developmental delay with recurrent episodes of metabolic decompensation, and asymptomatic forms identified by newborn screen (NBS) are also common (1-3). The characteristic symptoms of OAs, includes poor feeding, vomiting, failure to thrive, developmental delay, liver disease, neutropenia, thrombocytopenia, osteomalacia and osteoporosis, lethargy, hypotonia, seizures, ataxia, coma. Though clinical presentation varies greatly, severe and persistent metabolic acidosis of unexplained origin, elevated anion gap and severe neurologic manifestations, such as encephalopathy or seizures, remain strong diagnostic indicators of OA (1,4).
Twenty-five percent of human protein depends on the essential BCAAs—leucine, isoleucine, and valine, which compose 3 of the 9 essential AAs obtained through the diet, and are responsible for 35–40% of essential AAs in body protein and 14% of the total AAs found in skeletal muscle (2,4). The BCAAs share common membrane transport systems and enzymes for the first two steps in their catabolism, transamination to their respective ketoacids and irreversible oxidation to their coenzyme A (CoA) derivatives, which occur in the mitochondria (2). The many analogous, or even shared, enzymes between the converging catabolic pathways attributes to the characteristic clinical presentations that are common to OAs and other IEM (Figure 1).
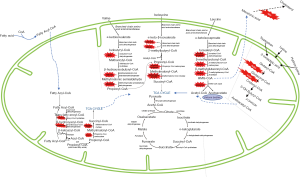
The ketoacids conversion to their CoA derivatives through oxidative decarboxylation and coupled thioesterification by branched chain ketoacid dehydrogenase (BCKD) complex, is irreversible (2,4). Multiple subunits, including E1, a thiamine pyrophosphate-dependent decarboxylase; E2, a lipoate-dependent transacylase; and E3, a dehydrogenase, make up the BCKD enzyme complex, which is similar in structure to both pyruvate and α-ketoglutarate dehydrogenases due to the common subunit (E3) that is shared by all 3 of the enzyme complexes (2,4).
BCKD plays a key role in nitrogen metabolism and, like its analogous dehydrogenases, it is tightly regulated by a kinase/phosphatase system. End-products of their metabolism, succinyl-CoA and/or acetyl-CoA, can then enter the Krebs cycle for energy production and gluconeogenesis, or to be recycled as acetyl-CoA and acetoacetate to serve as precursors for lipogenesis and synthesis of ketone bodies (2,4). In this way, the BCAAs can be glucogenic (valine), ketogenic (leucine) or both (isoleucine) (4).
All OAs can be diagnosed by gas chromatography-mass spectrometry (GC/MS) or tandem MS (MS/MS) identification of OA profiles of urine, serum, or cerebrospinal fluid (CSF), though they are present in highest concentration in the urine (1,2). Collectively, over 100 OAs can be identified in abnormal amounts in the urine due to OAs. GC/MS and tandem MS may also be used to clinically assess functional status, including mitochondrial energy production efficiency, neurotransmitter metabolism, functional vitamin, mineral and AA deficiencies, and metabolic disequilibrium and toxicity, particularly during episodes of metabolic decompensation. Other forms of analysis are available, such as capillary electrophoresis and high-resolution proton nuclear magnetic resonance, but are not widely used (1).
Early detection by the NBS with tandem MS allows for early intervention and better outcomes in all of the classical disorders of BCAA catabolism (2-4).
In this article, we will review some of the common IEMs associated with BCAA and lysine, tryptophan catabolism with emphasis on the neurodevelopmental aspects of the clinical presentation and course.
MSUD will not be reviewed in this article as covered in another article topic in this issue [AA disorders by Aliu et al. (5)].
IVA
Clinical
IVA was the first condition recognized as an OAD. Diagnosed in infants with acute episodic encephalopathy when the characteristic odor of “sweaty feet” was shown to be caused by an accumulation of unconjugated isovaleric acid due to a defect in isovaleryl-CoA dehydrogenase (IVD) (1-3,6).
IVA generally has two symptomatic forms and an asymptomatic form that is commonly picked up by NBS. The “acute neonatal form” is most common, and presents as an acute episode of fulminant metabolic ketoacidosis, accompanied by lethargy, poor feeding, vomiting, bone marrow failure, hyperammonemia, encephalopathy, and the characteristic “sweaty foot” odor. The “chronic intermittent” form presents later in childhood with developmental delay, with or without recurrent episodes of ketoacidosis during periods of catabolic stress (3,4,6).
Neurocognitive outcome is better associated with the age at diagnosis than with the number or severity of metabolic decompensations, suggesting that oxidative stress from chronic, persistent buildup of isovaleric acid is more neurologically detrimental than acute buildup. Early neonatal presentation is highly associated with mortality rate but, for infants that survive, early diagnosis is associated with far better outcomes than later diagnosis, even when diagnosed at symptomatic presentation rather than by NBS. IVA seems to have much milder effects on neurocognitive development, leading to a more favorable long-term outcome with 62% attaining normal or near normal neurocognitive functioning, and only 4% having severe developmental delay, consistent with past results (3).
Frequency of metabolic decompensation seems higher in the neonatal—infancy period and decreases with age by late childhood or adolescence; which may be secondary to acquired immunity to common childhood illnesses or infections. Other co-morbidities include failure to thrive, myeloproliferative syndrome, pancytopenia, seizure, liver dysfunction, cardiopulmonary disorders, pancreatitis, Angelman and Fanconi syndromes (1,6-10).
Metabolic findings and diagnosis
Metabolic ketoacidosis with an unexplained anion gap is typical in IVA (1). OA analysis by GC/MS is typically positive for isovalerylglycine and 3-hydroxyisovaleric acid. Other abnormal levels of metabolites can include lactic acid, 3-hydroxybutyric acid, acetoacetic acid, 3-hydroxyisovaleric acid, isovalerylglutamic acid (1,2).
IVA is caused by a deficiency of IVD, a mitochondrial enzyme which catalyzes the third step in leucine catabolism (dehydrogenation of isovaleryl-CoA to 3-methylcrotonyl-CoA) and subsequent buildup of isovaleryl-CoA and its derivatives. Isovaleryl-CoA is generally conjugated and excreted as nontoxic isovalerylglycine. However, during events which trigger episodes of metabolic decompensation, such as infection, pregnancy, protein ingestion, or breakdown with heavy dieting or exercise, the concentration of isovaleryl-CoA exceeds the liver’s esterifying capacity, resulting in accumulation of isovaleryl-CoA as isovaleric and 3-hydroxyisovaleric acids (2).
Isovaleryl-CoA functions as an N-acetyl-glutamate synthetase (NAGS) inhibitor leading to urea cycle impairment and hyperammonemia. Accumulation of isovalerylcarnitine (a C5 acylcarnitine) may also be identified with acylcarnitine analysis. Apart from biochemical analytes; IVD enzyme activity in leukocytes and cultured fibroblasts can also confirm diagnosis (1,2).
Patients with IVA can be detected by NBS prior to the onset of symptoms, and allows for significant reduction of symptom severity, morbidity and mortality through early intervention and appropriate preventative therapy. For this reason, early diagnosis by NBS is beneficial even for forms of IVA that do not manifest until later in childhood (3,4).
Treatment
Protein restriction to reduce the load of the toxic metabolites along with glycine and/or carnitine supplementation to promote the synthesis and excretion of less toxic conjugates continues along with use of leucine restricted metabolic formula seems to be the core treatment for OADs (3,4). Additionally, common childhood illness associated preventive measures and prompt use of acute illness or emergency protocol during metabolic decompensations prevent poor outcomes, reduce morbidity and mortality.
Genetics and epidemiology
IVA is an autosomal recessive disorder, due to mutations in IVD gene encoding IVD enzyme, located on chromosome 15q15.1 (2). NBS helped us understand that the common missense mutation p.A282 V, leading to increased metabolite levels in plasma and urine, but has little or no risk of clinical disease. While some forms remain asymptomatic and go undiagnosed even into adulthood (1,2). Other chromosome 15 disorders, such as Angelman syndrome, involving uniparental disomy (UPD) can be seen in rare cases of IVA (10).
PA
Clinical
Like the rest of the classical OAs, there are two PA clinical presentation—most common acute neonatal onset, and a late-onset chronic intermittent form. The neonatal onset occurs within the first few days of life beginning with ketoacidosis, poor feeding and vomiting, and then progresses to encephalopathy, lethargy, seizures, coma, and death. Late-onset PA develops chronically and presents with recurrent episodes of ketoacidosis similar to that of neonatal onset but with developmental regression, protein intolerance, chronic vomiting, failure to thrive, and hypotonia. Atypical presentations, such as movement disorders without metabolic decompensations, also occur (1,2,4,11,12).
Neurodevelopmental outcome is generally poor compared to that of IVA or MMA, with >70% of affected individuals reported to have cognitive deficit, movement disorders, and/or developmental delay in gross motor skill, fine motor skill and language skill. Attention deficit-hyperactivity disorder (ADHD), autism spectrum disorder (ASD) are also reported (12). Common neurological manifestations include seizures (most commonly generalized tonic-clonic), extrapyramidal symptoms, movement disorders, hallucinations and psychosis, and optic and brain atrophy. Basal ganglia lesions with stroke-like symptoms also occur, but are more common with MMA. These complications lead to poor neurocognitive outcomes, typically worse than IVA or MMA, with chronic intellectual disability and movement disorders (1-4,11,12).
Cardiac complications are more frequent in PA than MMA, and commonly include cardiomyopathy with or without acute heart failure, and arrhythmias, particularly prolonged QTc interval. Other late complications include recurrent pancreatitis, anorexia, failure to thrive, dermatitis and hearing loss (1,2,4,11,12).
Metabolic findings and diagnosis
Urine OA analysis and serum acylcarnitine profile help differentiate PA from other OAs, such as MMA, and metabolic diseases (1,4,12,13).
During metabolic crisis, urine OA analysis typically shows elevated 3-hydroxypropionic, 2-methylcitric acids (2-MCAs), propionylglycine, tyglylglycine, and the abnormal ketone bodies that result from inclusion of propionyl-CoA in place of acyl-CoA (1,2). Acylcarnitine analysis commonly can reveals increased glycine and propionylcarnitine (C3 carnitine). Other nonspecific metabolites can also be present (1,2,12). But, MCA presence anytime is a diagnostic marker (1).
Prenatal diagnosis of a PA can be made in the fetus by demonstrating deficiency of propionyl-CoA carboxylase (PCC) activity in cultured amniocytes or chorionic villus cells. NBS by tandem MS effectively identifies PA in neonates, though early diagnosis and intervention do not prevent neurodevelopmental manifestations, though it usually decreases severity (4,12). Current therapy options seem inadequate in preventing long-term complications. Diagnostic confirmation is achieved by DNA sequencing of PCCA and PCCB genes or, less commonly, by enzyme assay of cultured leukocyte or fibroblasts (1,2,12).
Treatment
Treatment approaches for PA include sufficient caloric and non-propiogenic protein intake to support normal growth and development. This can often be accomplished by formulas devoid of propiogenic AAs valine, isoleucine, methionine, and threonine (2,4,11,14). Dietary restriction of odd-chain fatty acids, another propionic acid precursor, may also be needed (12).
During metabolic crisis, acute illness protocol with parenteral caloric sources and slow transition to non-propiogenic protein intake can prevent further protein catabolism. Additionally, carglumic acid (“carbaglu”) as an ammonia scavenger helps acute hyperammonemia resulting from urea cycle NAGS inhibition (2,12,14,15).
Carnitine supplementation can address secondary carnitine deficiency, caused by increased excretion of propionyl-CoA as propionylcarnitine, a less toxic metabolite (2,4,12,14).
Intermittent treatment with antibiotics with poor enteral absorption, such as neomycin or metronidazole, can be used to decrease the propionic acid produced by bowel flora and absorbed in the gastrointestinal (GI) tract (2,11,16).
Ultimately, liver transplantation can be used to decrease the frequency and severity of metabolic decompensations, though catabolic ketoacidosis and related neurological events may still recur with propionic acid accumulation (2,4,11,12).
Genetics and epidemiology
PA is one of the most common life-threatening OAs, with a global incidence of about 1 in 100,000 live births (11,17). It is an autosomal recessive disorder due to mutations in PCCA gene on chromosome 13q32.3 and PCCB gene on chromosome 3q22.3. These lead to deficient PCC activity, a mitochondrial biotin-dependent carboxylase enzyme. PCC is a heterododecamer with six α-subunits (encoded by PCCA gene) that contain biotin binding sites, and six β-subunits (encoded by PCCB gene) (1,2). PCC help conversion of propionyl-CoA to methylmalonyl-CoA in the catabolism of VOMIT.
MMAs
Clinical
MMA disorders are a group of IEM caused by deficient activity of methylmalonyl-CoA mutase (MCM) and/or PCC, enzymes of the propionyl-CoA catabolic pathway, leading to accumulation of propionic acid and/or methylmalonic acid. These disorders can present as an isolated defect in propionyl-CoA catabolism, or in combination with other inherited metabolic defects (1,2,11).
Isolated MMA has varying phenotypes with wide ranges of onset and severity. The acute/neonatal form is most common and presents within the first few days of life with life-threatening ketoacidosis, hyperammonemia, hyperglycinemia, hypoglycemia, vomiting, respiratory distress, lethargy, hypotonia, hypothermia, neutropenia and thrombocytopenia. In the infantile/B12-unresponsive phenotype, infants are normal at birth, but develop lethargy, vomiting, dehydration, hypotonia, hepatomegaly, encephalopathy, and failure to thrive within a few weeks to months after birth, usually triggered by protein load when feeding is initiated. The intermediate B12-responsive phenotype generally presents in the first few months to years of life with developmental delay, hypotonia, anorexia, sometimes with protein aversion and/or vomiting and lethargy after protein intake, and failure to thrive. Atypical and “benign” adult phenotypes are usually diagnosed by NBS, or later identified incidentally, but typically remain asymptomatic with only mildly increased urinary methylmalonate. All of the phenotypes are associated with intermittent episodes of metabolic decompensation, which can resemble diabetic ketoacidosis (DKA) or septic shock, triggered by hypercatabolic states, such as infections, stress, pregnancy, and intense dieting and exercise (1,2,4,11,18,19).
The most common long-term manifestations of MMA are developmental delay and neurocognitive impairment. With the exception of vitamin B12-responsive subtypes, MMA is associated with poorer neurodevelopmental and overall long-term outcomes than IVA, commonly resulting in early death or severe neurocognitive impairment (3,11). “Metabolic stroke” with acute and chronic lesions of the basal ganglia, particularly the globus pallidus can also occur, but is more common with PA, and which may be associated with a disabling movement disorder with choreoathetosis, dystonia, and other extrapyramidal symptoms, and/or spastic para/quadriparesis. Other movement disorders may also be present, but are still more common in PA (4,11).
Additionally, individuals with MMA are likely to develop tubulointerstitial nephritis with progressive renal failure—a complication that is almost exclusive to MMA (1,3,4,11,18,20).
Other common complications are similar to those seen with PA, and include recurrent pancreatitis, osteoporosis, cardiopulmonary disorders, neutro- and pancytopenias with functional immune impairment, growth failure, and failure to thrive (1-4,11,18). Less common complications include dermatitis, hearing loss, optic neuropathy and “bullseye” visual field defects (4,11,18).
MMA with homocystinuria (MMA-HC) is a combined disorder that typically presents acutely with failure to thrive, hypotonia and other neurodevelopmental complications, and hematologic abnormalities, such as macrocytic anemia and megaloblastosis. Hemolytic-uremic syndrome can also occur but is less common (1,2,11,20). Combined malonic aciduria and MMA (CMAMMA) disorders are typically mild (2). More detailed reviews on intracellular cobalamin metabolism disorders with MMA findings can be found in GeneReviews (21).
Metabolic findings and diagnosis
MCM converts of L-methylmalonyl-CoA to succinyl-CoA, and is a key enzyme in catabolism of VOMIT and cholesterol side chains (1,2,4,11,18). MMA disorders can result directly from defects in MCM, or indirectly from inhibition of MCM activity, both of which ultimately result in accumulation of methylmalonic acid in many tissues and body fluids (1,2,4,18).
In addition to urine/plasma OA analysis for diagnosis of suspected MMA, 14C-propionate incorporation studies; vitamin B12 responsiveness and distribution assays; and plasma levels of homocysteine, malonic acid, and cobalamin, are important for excluding differential diagnoses, including PA and acquired vitamin B12 deficiency, and for distinguishing between isolated MMA subtypes and combined disorders (1,2,4,11,18,22).
Urinary OA analysis shows elevated methylmalonic acid, especially in subtypes resulting from MCM defects, as well as elevated tiglylglycine, a metabolic derivative from the isoleucine pathway, 3-hydroxypropionic acid, 2-MCA, and the same ketone bodies identified in PA. The elevations in 3-hydroxypropionic and 2-MCA occur due to compensatory activation of propionyl-CoA oxidation pathways secondary to inhibition of PCC (1,2,11,17,18). Glycine and propionylcarnitine are also elevated, but less than in PA (2).
Diagnosis of MMA is generally confirmed by DNA sequencing of associated genes, with confirmation of carrier status in the parents, and less commonly by complementation analysis of cultured patient fibroblasts (1,2,11,18).
Elevated 2-MCA, 3-methylglutaconic acid (3-MGA), 3-hydroxyisovaleric acid, succinate, fumarate, 2-oxoglutaric acid, and succinylcarnitine esters may also be increased in patients with a succinyl-CoA ligase (SUCL) defect (1,2,4).
NBS with tandem MS is effective in identifying MMA, though the benefit of NBS and subsequent clinical intervention is not as clear for MMA as it is for IVA. For defects that lead to milder isolated and combined presentations, such as partial MCM deficiency (mut−) and defects of cobalamin A and B, early diagnosis by NBS may positively impact the clinical course. However, NBS may be less beneficial in the most severe, early-onset forms of MMA, with immense metabolite accumulation and hyperammonemia before NBS is done or NBS results availability, resulting in significant brain damage or even death before intervention can be attempted (4,11,18).
Treatment
The treatment approaches of MMA disorders are the same as those for PA as noted above, with the addition of vitamin B12/cobalamin supplementation for the B12-responsive subtypes of MMA, which are further discussed below (1,2,4,11).
Accompanying conditions in other presentations of MMA are not addressed in this article and can be found in GeneReviews (21).
Genetics and epidemiology
Before specific gene mutations were identified, many defects in B12 metabolism which lead to MMA were classified using genetic complementation methods, and include Mut, Cbl types A, B, C, D, Dv2, F, J, and X (2,20,21). The mutations associated with these defects follow autosomal recessive inheritance, except CblX, which is an X-linked mutation in the HCFC1 transcription factor encoded by the CBLC gene. Few labs continue to use diagnostic enzyme complementation (1,2).
Isolated MMA is associated with mutations in 1 of 5 genes, MMUT, MMAA, MMAB, MCEE, and MMADHC, with MMUT being the most common. Direct mutations in MMUT, can cause complete (mut0) or partial (mut−) deficiency of MCM, resulting in phenotypes that are unresponsive to vitamin B12 supplementation. Deficient cofactor 5'-deoxyadenosylcobalamin (AdoCbl), which is required for function of MCM, is associated with the vitamin B12-responsive cblA, B, and D complementation groups, encoded by MMAA, MMAB, and MMADHC, respectively. Deficiency of methylmalonyl-CoA epimerase, encoded by MCEE, is a very rare form of isolated MMA (1,2,4,18).
Combined MMA presentations can be seen with homocystinuria, malonic aciduria, mitochondrial DNA depletion syndrome, and deficient methylmalonate semialdehyde dehydrogenase (MMSDH) or cell surface transcobalamin receptor (CD320) (1,18).
Combined MMA and homocystinuria (MMA-HC) disorders arise from other defects in cobalamin metabolism involving several genes, including MMACHC, MMADHC, LMBRD1 (a lysosomal membrane transporter), and ABCD4. Mutations in LMBRD1 and ABCD4 result in failure of lysosomes to release vitamin B12. These autosomal recessive mutations cause impaired hepatic conversion of dietary cobalamin to methylcobalamin and adenosylcobalamin, resulting in decreased activity of MCM and methionine synthase (1,2,4,18).
CMAMMA disorders can result from deficiencies in various pathways. Causative defects have been identified in members of the acyl-CoA synthetase family, most commonly member 3 (ACSF3), a methylmalonyl- and malonyl-CoA synthetase that produces the malonyl-CoA required for fatty acid synthesis. Plasma and urinary OA analysis detects elevations of both malonic acid and methylmalonic acid (1). Deficiency of malonyl-CoA decarboxylase has also been tied to MMA or CMAMMA with markedly increased malonic acid, but only small elevations of methylmalonic acid (2).
Mitochondrial DNA depletion resulting in loss of the (SUCL subunits SUCLA2, SUCLG1, and SUCLG2 of the succinyl-CoA synthetase complex is a mechanism outside methylmalonic catabolism that has been shown to cause forms of mild to moderate MMA (1,2,18).
The incidence of MMA in the general population was estimated to be about 1 in 50,000 (11).
Glutaric acidemia type I (GA-I)
Dysfunction in the mitochondrial matrix enzyme, glutaryl-CoA dehydrogenase (GCDH), necessary for conversion of glutaryl-CoA to crotonyl-CoA in the catabolism of AAs lysine, hydroxylysine and tryptophan leads to accumulation of glutaric acid—a potent neurotoxin leading to the neurodegenerative presentation of GA-I (23).
Clinical
Typical findings include early infancy onset of macrocephaly with delay in developmental milestone achievements. Progression of condition in untreated patient culminate in progressive dystonia and dyskinesia in toddlerhood. Common childhood illnesses/stress can trigger acute and rapid metabolic decompensation leading to encephalopathy and devastating striatal injury even in treated patients, making GA-I a difficult NBS condition to manage. Once basal ganglia injury occurs, neurological symptoms remain static mimicking extrapyramidal cerebral palsy. Often, findings of subdural hematoma resulting from decreasing cerebral parenchymal volume and stress/tearing of the bridging veins along with retinal hemorrhage can be confused with non-accidental trauma or child abuse, posing socio-legal disadvantage to an otherwise loving family.
Metabolic findings and diagnosis
As seen in other OADs, high anion gap metabolic ketoacidosis, hyperammonemia, pancreatic enzyme abnormalities, elevated CSF proteins; with elevation in saccharopine; low free carnitine as non-specific biomarkers. While increase in glutarylcarnitine and characteristic OAs such as glutaric, 3-OH glutaric and glutaconic acids are the specific biomarkers. These elevations are direct result of faulty GCDH dehydrogenation and decarboxylation activity required for glutaryl-CoA and glutaconyl-CoA conversion to crotonyl-CoA in the lysine, hydroxylysine and tryptophan catabolism in the mitochondrial matrix (Figure 1). GCDH enzyme analysis in leukocytes and fibroblasts can help diagnosis but molecular analysis of GCDH gene can with prognostic and management approaches. NBS detection of glutarylcarnitine (C5DC) helps early detection of GA-I though identification of derivatized or underivatized C5DC did not seem to affect diagnostic confirmation (24).
Treatment
Main treatment approaches as noted in other OAD is restricted offending AA metabolic formula, L-carnitine supplementation and prompt implementation of acute illness protocol with parenteral caloric supplementation with common childhood illnesses or stressors with ultimate goal of striatal/basal ganglia injury prevention.
Genetics and epidemiology
GA-I is an autosomal recessive disorder, caused by mutations in the GCDH gene, on chromosome 19p13.13, encoding the GCDH enzyme. GA-I is a commonly associated condition within Older Order Amish community in Pennsylvania, USA. GA-I global prevalence is 1:100,000 but a more recent US NBS data in found it to be 1:89,438 (24).
3-methylglutaconic acidurias/acidemias (3MGA)
Clinical
This is a group of disorders of various causes which collectively lead to accumulation and excretion of 3-MGA. Traditionally there have been five subtypes, though, more recent classification divides them into primary and secondary causes (2). Clinical features and age of onset are remarkably variable, and include cardiomyopathy, optic atrophy, Leigh syndrome, hyperammonemia, and lactic acidemia (2,4).
Metabolic findings and diagnosis
Urinary OA analysis shows elevated 3-methylglutaconic (3-MGC) and 3-methylglutaric acids, derivatives of the 3-MGC-CoA that accumulates due to deficient 3-methylglutaconic-CoA hydratase activity. Elevated 3-hydroxyisovaleric acid and normal 3-hydroxy-3-methylglutaric acid are also present (2,4). Enzyme activity of 3-MGC-CoA hydratase (encoded by the AUH gene) can be measured in fibroblasts (2).
Treatment
There is some evidence to support dietary leucine restriction and supplementation with L-carnitine, although treatment remains mostly supportive (2,4).
Genetics and epidemiology
In addition to type I/primary 3MGA, several pathogenic variants have been identified as secondary causes, including type II/Barth syndrome (TAZ gene), type III/Costeff optic atrophy syndrome (OPA3 gene), MEGDEL syndrome (SERAC1 gene), type V/dilated cardiomyopathy with ataxia syndrome (DNAJC19 gene), and type IV, including defects in the TMEM70 gene and disorders of unknown origin (2,4). Other OADs affecting isoleucine catabolism are 2-methyl-bytyryl-CoA dehydrogenase [aka. short/branched chain acyl-CoA dehydrogenase (SBCAD)] deficiency; 2-methyl-acetoacetyl-CoA thiolase deficiency. Other OADs affecting valine catabolism are isobutyryl-CoA dehydrogenase (IBD) deficiency. These can be reviewed in omim (www.omim.org), GeneReviews and Scriver’s OMMBID.
Nutrition management of organic acidemias
The goal of nutrition management for a patient with an organic acidemia is to reduce the accumulation of toxic metabolites, provide adequate nutrients to maintain normal growth and development, maintain normal plasma AA concentrations, and prevent catabolism. Metabolic nutritional formula that restricts propiogenic AAs and medications/supplements such as Levocarnitine (binds with toxic acyl-CoA metabolites); nitrogen scavengers (during hyperammonemia); biotin (cofactor for use in PA); IM-hydroxycobalamin (cofactor for use in MMA); and riboflavin and pantothenic acid (cofactors for use in GA-1) are main stay of management. Individualized metabolic nutrition prescriptions determined by metabolic dietitian to balance the use of propiogenic AA-restricted medical foods (Table 1) combined with a prescribed amount of intact protein (breast milk, standard infant formula, meat, eggs, dairy etc.) are necessary for optimal growth and development.

Full table
A current shift in the nutrition management of organic acidemias, especially MMA and PA, involve maximizing intact protein tolerance instead of over reliance on medical foods (25).
The nutrition management of GA-1 is additionally challenging because good biomarkers to guide treatment have not been identified and there is lack of agreement for how long and to what degree to restrict propiogenic AAs as the patients age through childhood (26).
Metabolic decompensation with any illness requires aggressive management using intravenous fluids with 10% dextrose at 1.5 times maintenance plus 20% Intralipid® at 2 g/kg to prevent catabolism. During illness, propiogenic AA intake will elevate ammonia further and is therefore eliminated or reduced to 50% for 24–48 hours. Poor metabolic control can cause loss of appetite and many patients require a nasogastric (NG) tube upon admission to start enteral nutrition. Protein-free calories are increased to meet patient’s energy needs to suppress catabolism. After 24–48 hours, it is important to reintroduce enteral intact protein and medical food because over restriction of protein can result in endogenous protein catabolism and refractory hyperammonemia. If oral or enteral intake is difficult, parenteral nutrition with 10% AAs can meet necessary protein needs.
Most metabolic clinics trend diagnostic biochemical analytes for individual OA as noted above apart from plasma AAs, acylcarnitine profile, OAs, and several other micronutrients on a routine basis to adjust management.
Thoughts on future direction
Much of the pathology in disorders of BCAA metabolism is known to be caused by oxidative damage by reactive oxygen species (ROS). Continued research is needed to identify additional therapies, with a particular focus on possible targeted therapies for mitochondrial dysfunction and the associated oxidative damage seen in the OAs (1,4,11,17).
In addition, gut microbiome plays a key role in the amount of BCAAs absorbed, thus treatments for dysbiosis of the gut microbiome may play an increasing role in OAD management. Few studies have been published regarding the relationship between the microbiome and clinical manifestations of OAs, which opens up the possibility of specific therapeutic probiotics being used in the future to help manage the metabolic derangements (27,28).
The majority of people with OADs are affected by neurodevelopmental manifestations, most commonly cognitive deficit and developmental delays in gross motor, fine motor, and language skills, ADHD and ASD make integrated behavioral health (IBH) model clinic important for best long-term outcome and treatment compliance.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Villani GR, Gallo G, Scolamiero E, et al. "Classical organic acidurias": diagnosis and pathogenesis. Clin Exp Med 2017;17:305-23. [Crossref] [PubMed]
- Vockley J. Disorders of Branched Chain Amino and Organic Acid Metabolism. In: Kline MW. editor. Rudolph’s Pediatrics. 23 edition. New York, NY: McGraw-Hill Education, 2018.
- Grünert SC, Wendel U, Lindner M, et al. Clinical and neurocognitive outcome in symptomatic isovaleric acidemia. Orphanet J Rare Dis 2012;7:9. [Crossref] [PubMed]
- Manoli I, Venditti CP. Disorders of branched chain amino acid metabolism. Transl Sci Rare Dis 2016;1:91-110. [Crossref] [PubMed]
- Aliu E, Kanungo S, Arnold GL. Amino acid disorders. Ann Transl Med 2018. In press. [Crossref]
- Najafi R, Hashemipour M, Mostofizadeh N, et al. Demographic and Clinical Findings in Pediatric Patients Affected by Organic Acidemia. Iran J Child Neurol 2016;10:74-81. [PubMed]
- Qadi AM, Hamadah HK, Jijeh AM, et al. Ebstein cardiac anomaly, functional pulmonary atresia and isovaleric acidemia: A case report. J Saudi Heart Assoc 2014;26:170-3. [Crossref] [PubMed]
- Mantadakis E, Chrysafis I, Tsouvala E, et al. Acute pancreatitis with rapid clinical improvement in a child with isovaleric acidemia. Case Rep Pediatr 2013;2013:721871. [Crossref] [PubMed]
- Sag E, Cebi AH, Kaya G, et al. A Rare Cause of Recurrent Acute Pancreatitis in a Child: Isovaleric Acidemia with Novel Mutation. Pediatr Gastroenterol Hepatol Nutr 2017;20:61-4. [Crossref] [PubMed]
- Lambrecht A, Pichard S, Maurey H, et al. Angelman syndrome and isovaleric acidemia: What is the link? Mol Genet Metab Rep 2015;3:36-8. [Crossref] [PubMed]
- Baumgartner MR, Hörster F, Dionisi-Vici C, et al. Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphanet J Rare Dis 2014;9:130. [Crossref] [PubMed]
- Wongkittichote P, Ah Mew N, Chapman KA. Propionyl-CoA carboxylase - A review. Mol Genet Metab 2017;122:145-52. [Crossref] [PubMed]
- Aldubayan SH, Rodan LH, Berry GT, et al. Acute Illness Protocol for Organic Acidemias: Methylmalonic Acidemia and Propionic Acidemia. Pediatr Emerg Care 2017;33:142-6. [PubMed]
- Fraser JL, Venditti CP. Methylmalonic and propionic acidemias: clinical management update. Curr Opin Pediatr 2016;28:682-93. [Crossref] [PubMed]
- Valayannopoulos V, Baruteau J, Delgado MB, et al. Carglumic acid enhances rapid ammonia detoxification in classical organic acidurias with a favourable risk-benefit profile: a retrospective observational study. Orphanet J Rare Dis 2016;11:32. [Crossref] [PubMed]
- Rafique M. Emerging trends in management of propionic acidemia. Arq Bras Endocrinol Metabol 2014;58:237-42. [Crossref] [PubMed]
- Richard E, Gallego-Villar L, Rivera-Barahona A, et al. Altered Redox Homeostasis in Branched-Chain Amino Acid Disorders, Organic Acidurias, and Homocystinuria. Oxid Med Cell Longev 2018;2018:1246069. [Crossref] [PubMed]
- Manoli I, Sloan JL, Venditti CP. Isolated Methylmalonic Acidemia. GeneReviews®. University of Washington, Seattle, 1993.
- Saini N, Malhotra A, Chhabra S, et al. Methylmalonic acidemia mimicking diabetic ketoacidosis and septic shock in infants. Indian J Crit Care Med 2015;19:183-5. [Crossref] [PubMed]
- Liu J, Peng Y, Zhou N, et al. Combined methylmalonic acidemia and homocysteinemia presenting predominantly with late-onset diffuse lung disease: a case series of four patients. Orphanet J Rare Dis 2017;12:58. [Crossref] [PubMed]
- Sloan JL, Carrillo N, Venditti CP, et al. Disorders of Intracellular Cobalamin Metabolism [Internet]. GeneReviews®. University of Washington, Seattle, 1993.
- Keyfi F, Talebi S, Varasteh AR. Methylmalonic Acidemia Diagnosis by Laboratory Methods. Rep Biochem Mol Biol 2016;5:1-14. [PubMed]
- Goodman SI, Frank F. Organic Acidemias Due to Defects in Lysine Oxidation: 2-Ketoadipic Acidemia and Glutaric Acidemia | The Online Metabolic and Molecular Bases of Inherited Disease | OMMBID | McGraw-Hill Medical. In: Valle D, Beaudet AL, Gibson K, et al. editors. The Online Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill, 2014.
- Kanungo S, Feuchtbaum L, Sermba L, et al. Glutaric Aciduria Type 1 (GA-I). California newborn screening (NBS) program experience on detection, with and without derivatization, and long term clinical outcome. 12th ICIEM (2013), Barcelona, Workshop 5; W-017: Outcome of patients detected through expanded newborn screening. J Inherit Metab Dis 2013;36:55. Available online: https://doi.org/ [Crossref]
- Manoli I, Myles JG, Sloan JL, et al. A critical reappraisal of dietary practices in methylmalonic acidemia raises concerns about the safety of medical foods. Part 1: isolated methylmalonic acidemias. Genet Med 2016;18:386-95. [Crossref] [PubMed]
- Bernstein LE, Rohr F, Helm JR. editors. Nutrition Management of Inherited Metabolic Diseases. Cham: Springer International Publishing, 2015.
- Colonetti K, Roesch LF, Schwartz IVD. The microbiome and inborn errors of metabolism: Why we should look carefully at their interplay? Genet Mol Biol 2018;41:515-32. [Crossref] [PubMed]
- Wishart DS. Emerging applications of metabolomics in drug discovery and precision medicine. Nat Rev Drug Discov 2016;15:473-84. [Crossref] [PubMed]


