The nexus of functional exercise capacity with health-related quality of life in lung cancer: how closely are they related?
Lung cancer is a devasting and common diagnosis worldwide. Approximately 81% of patients present once the cancer has spread beyond the primary site and consequently surgical treatment is often not an option, with chemoradiation, targeted therapy or immunotherapies being most common in stage III to IV disease. Lung cancer has a high disease burden with symptoms such as shortness of breath, cough and fatigue that significantly impact the patient’s activities of daily living. In the last 10 years clinicians and researchers have focused on measuring symptoms and patient reported outcomes, and providing interventions to improve these. Predominate in these measures are functional exercise capacity and health related quality of life (HRQoL). Functional exercise capacity can be measured in the laboratory as a gold standard using cardiopulmonary exercise testing (CPET) or by using field walking tests such as the six-minute walk test (6MWT). The field walking tests are easier and cheaper to perform clinically, and are used in many chronic disease populations to assess and measure change in functional exercise capacity. Functional exercise capacity is an important variable for patients as it is shown to be highly associated with an individual’s ability to undertake activities of daily living. Closely related to this is an individual’s HRQoL which measures several domains related to the ability to participate in home and community activities and relationships. Improving HRQoL is the ultimate goal of any rehabilitation or exercise program in all chronic disease states. There is a significant body of research that has presented conflicting information on the ability of exercise interventions to improve HRQoL in patients with lung cancer, due in part to the different measurement timepoints used, the sample heterogeneity, and the fact that there may be a response shift in patient’s perception of their HRQoL across time from diagnosis. It is reasonable, and accords with common sense, that functional exercise capacity is related to HRQoL. It is in this context that we review the relationship of functional exercise capacity to HRQoL in patients with lung cancer using the paper by Ha and colleagues (1) and discuss the wider implications and research related to this important topic.
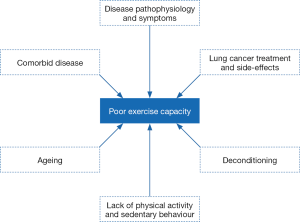
The 6MWT is a commonly used measure of functional exercise capacity in the lung cancer population (2). Performance in the 6MWT is an important outcome in lung cancer, given its association with post-operative complications, HRQoL and survival (2). The study by Ha and colleagues demonstrated impairments in the 6MWT in patients a median of 19 months following curative lung cancer treatment (1). The mean [standard deviation (SD)] distance walked in the test by the group was only 335 m [126] which corresponds to 65% of the groups predicted distance based on normative reference calculations which take into consideration the participants’ sex, age, height and weight (3). The magnitude of impairment may have been impacted by the relatively high rate of participants stopping during the test (26%) due to symptom limitations, combined with the fact that there was not a practice 6MWT trial, which is known to result in higher distances walked in the second repeat test (4). Despite these issues, prior studies have similarly shown impairments in functional exercise capacity in lung cancer, albeit in smaller samples (5-7). The cross-sectional nature of the study by Ha and colleagues (1) means there is no available data on functional exercise capacity in the cohort, either before lung cancer treatment or after treatment in a longitudinal manner in order to determine any pattern of change over time. Prior work by our team and others (5,8) demonstrated that functional exercise capacity is lower than predicted in patient’s pre-treatment (84% predicted), and it deteriorates further after treatment (69% predicted at 6 months following curative treatment) (5). The mechanisms for impairment and deterioration in functional exercise capacity in lung cancer are multi-factorial as shown in Figure 1 (9-12). These factors, including the combination of cancer symptoms and treatment side-effects, combine to accelerate this ‘perfect deconditioning storm’ reducing either the body’s ability to deliver and/or utilize oxygen leading to reduced exercise capacity (13,14). Reduced exercise capacity and muscle dysfunction predict poor postoperative outcomes (15) and are strong predictors of HRQoL (14). Further, in lung cancer, emerging evidence suggests that loss of whole-body muscle mass may be a significant contributor to morbidity (13,15-17).
Indeed, in the study by Ha and colleagues, 15 participants stopped during the 6MWT due to symptoms (pain, dyspnoea or fatigue) (1). Another study reported a third of participants stopped exercise tests early due to muscular fatigue (18). Recently, Burtin and colleagues demonstrated that quadriceps endurance is an independent contributor to performance of the 6MWT in lung cancer (19), highlighting the critical importance of peripheral muscle function as a potential contributor to impairments in functional exercise capacity. Given the heterogeneity of contributing factors leading to poor exercise capacity in lung cancer, detailed assessment and patient profiling of exercise limitations is essential as this will facilitate decision making around the choice of intervention(s) to address limitations (11).
Ha and colleagues sought to determine the specific association between the 6MWT and cancer-specific HRQoL measured by the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 summary score (1). Results demonstrated only a weak correlation (r2=0.16, P=0.001). More interestingly though, were the results of the multivariable linear regression analysis, that produced a final model containing 6MWT, heart failure, obstructive sleep apnea and psychiatric illness, which was independently associated with cancer-specific HRQoL (partial r2=0.20, P=0.001). It is not surprising that functional exercise capacity contributes to measures of HRQoL. Lack of sufficient tissue oxygen supply reduces exercise capacity and physical function leading to impairments in abilities to participate in activities of daily living (20,21). Measuring exercise capacity provides evidence about aerobic exercise capacity, a fundamental physiological determinant of lifestyle (22). It is clear then that exercise capacity would also be associated with the functional domains of HRQoL as indicated in the paper by Ha and colleagues. However, global HRQoL is a much broader construct, influenced by many factors such as other comorbid diseases, social situation and mental health as demonstrated in their study. The fact that their variables accounted for only 20% of the variance in HRQoL demonstrates well that HRQoL is a multifaceted construct but the conclusion in the paper by Ha and colleagues does not recognize the other important contributions of their model, nor other potential contributors not measured in the paper.
The importance of collecting patient-reported outcome measures (PROMs), in addition to routinely collected clinical data, is being increasingly recognised to enhance outcomes for patients. Basch and colleagues report improvements in HRQoL, treatment tolerance and survival, and reduced healthcare utilisation associated with routine symptom self-monitoring for people with metastatic cancer, including lung cancer (23). In addition to collecting data on HRQoL, Ha and colleagues also collected PROMs which are frequently impacted by a lung cancer diagnosis and subsequent treatment including: mood [Hospital Anxiety and Depression Scale (HADS)], fatigue (Brief Fatigue Inventory), sleep quality (Pittsburg Sleep Quality Index) and dyspnea [University of California San Diego Shortness of Breath Questionnaire (UCSD SOBQ)]. The authors report significant correlations between functional exercise capacity (6MWD) and depression (P=0.05), dyspnea (P=0.01) and fatigue (P=0.01). The prevalence of mood disorders reported in people with lung cancer is variable and may relate to the timing of measurement along the continuum of cancer care. Ha and colleagues report borderline anxiety and depression scores (> seven on the HADS subscales) in 19% and 31% of participants respectively. Recent work in a sample of 830 lung cancer survivors reports similar rates of borderline anxiety (21%) and higher rates of borderline depression (39%) (24). Abnormal levels of fatigue (scores >40) were also reported in 42% of participants in the study by Jung and colleagues (24), compared to only 24% reported by Ha and colleagues. Hospitalised patients prior to commencing treatment report high levels of anxiety and depression (65% for both anxiety and depression) (25). Rates continue to be higher than reported by Ha and colleagues in the period 3–6 months post-diagnosis (26,27). Poor sleep quality was reported by the majority of participants (73%), and the average score was only slightly lower than previously reported baseline values of participants recruited to home (28) or hospital-based physical activity programs (29). Supporting the authors’ remarks that participant comorbidities were likely well managed, is the observation that despite 77% of the sample reporting chronic obstructive pulmonary disease (COPD) or asthma, Borg ratings of dyspnoea pre and post 6MWT were low. The measure of dyspnoea indicated that only 26% of the sample had abnormal dyspnoea scores and this finding is in contrast to data reported from a high-risk surgical population two years post treatment (30). It appears that the PROMs reported within the study by Ha and colleagues, and the performance on 6MWT, are somewhat inconsistent with prior work (PROMs were commonly better in the study by Ha and colleagues). This finding is surprising given the high rate of reported comorbidities which commonly impact on PROMs such as symptoms and mood.
We note with interest the significant proportion of included participants with comorbid conditions, in particular hypertension (82%), hyperlipidaemia (82%) and (COPD)/asthma (77%) (1). High frequencies of comorbidities are also reported in long-term survivors of other forms of cancer. Leach and colleagues sampled 1,527 survivors of breast, colorectal, prostate and gynaecological cancers who averaged five comorbidities ever diagnosed and 1.9 diagnosed post-cancer. Importantly, amongst other factors physical inactivity was associated with a greater number of comorbidities occurring post-cancer diagnosis (31). The presence of comorbid chronic disease and physical activity levels has been previously reported to be significantly associated with HRQoL in a cross-sectional survey of 701 lung cancer survivors (32). Ha and colleagues report no significant association was found between comorbidities and the 6MWD, however acknowledge that the small sample size may be partly responsible for the lack of association.
The evidence supporting exercise training for patients with early stage lung cancer is now well-established and strong. Evidence for the role of exercise in later stage lung cancer is emerging but not yet established. The impairments demonstrated in the study by Ha and colleagues (1), including poor functional exercise capacity, HRQoL and cancer symptoms including dyspnoea, fatigue, anxiety and depression, may be able to be improved with specific exercise training. The participants in the current study were measured at varied time periods after their treatment [median 19 months, interquartile range (IQR), 4–44 months]. There is still a lack of data on the optimal timing for exercise training relative to treatment (9,33). With positive benefits seen in the pre-treatment (prehabilitation), early post-treatment and later post-treatment settings (34,35). Certainly, most of the studies conducted to date have included patients within the first year of their diagnosis and there is no rationale in general to be waiting to prescribe exercise beyond restoration of any acute medical issues post treatment. The rationale for exercise in the pre-treatment setting is to train patients to improve their cardiorespiratory fitness, with the view to prevent complications and lessen impairments following treatment (34). Whereas, the rationale for exercise in the post-treatment setting is to target impairments that may have occurred since treatment/diagnosis to facilitate the patient back to premorbid (pre-treatment) status (35). In the study by Ha and colleagues, we do not have information on usual care provided to participants, including whether any exercise training or advice was given (1). However, it may be safe to assume exercise training or advice was minimal, as usual care in most centres around the world is unlikely to include a focus on exercise at this time. Indeed, we could be confident that exercise training may be an appropriate modality for many of the participants to address their current issues. As is recommended in oncology exercise programs, exercise prescription should be tailored to the individual participant, following a thorough patient assessment to determine specific impairments and medical safety to exercise, and with consideration of the impact of other comorbidities (36). In the future we need data to inform the optimal timing for exercise training commencement. However, an immediate challenge for patients is access to exercise programs (10,37). This is because oncology exercise programs are not readily available throughout many countries in the world, and therefore encouraging patients to access programs when and where available should be a priority until these data are available to change our practice.
Acknowledgements
Funding: This work was supported by the Victorian Cancer Agency (Clinical Research Fellowship) to CL Granger. This work was supported by the Victorian Government Olivia Newton John Cancer Wellness and Research Centre Supportive Care PhD scholarship, through the Victorian Cancer Agency (ONJ16010) to L Edbrooke.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ha D, Ries AL, Mazzone PJ, et al. Exercise capacity and cancer-specific quality of life following curative intent treatment of stage I-IIIA lung cancer. Support Care Cancer 2018;26:2459-69. [Crossref] [PubMed]
- Granger CL, Denehy L, Parry S, et al. Functional capacity, physical activity and muscle strength of individuals with non-small cell lung cancer: A systematic review of outcome measures and their measurement properties. BMC Cancer 2013;13:135. [Crossref] [PubMed]
- Enright PL, Sherrill D. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med 1998;158:1384-7. [Crossref] [PubMed]
- Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014;44:1428-46. [Crossref] [PubMed]
- Granger CL, McDonald C, Irving L, et al. Low physical activity levels and functional decline in individuals with lung cancer. Lung Cancer 2014;83:292-9. [Crossref] [PubMed]
- Cavalheri V, Jenkins S, Cecins N, et al. Impairments after curative intent treatment for non-small cell lung cancer: a comparison with age and gender-matched healthy controls. Respir Med 2015;109:1332-9. [Crossref] [PubMed]
- Deslauriers J, Ugalde P, Miro S, et al. Long-term physiological consequences of pneumonectomy. Semin Thorac Cardiovasc Surg 2011;23:196-202. [Crossref] [PubMed]
- Jones LW, Hornsby W, Goetzinger A, et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer 2012;76:248-52. [Crossref] [PubMed]
- Granger CL. Physiotherapy management of lung cancer. J Physiother 2016;62:60-7. [Crossref] [PubMed]
- Granger CL, Connolly B, Denehy L, et al. Understanding factors influencing physical activity and exercise in lung cancer: a systematic review. Support Care Cancer 2017;25:983-99. [Crossref] [PubMed]
- Maddocks M, Granger C. Lower limb muscle function and exercise performance in lung cancer. Respirology 2017;22:1053-4. [Crossref] [PubMed]
- Granger CL, Parry SM, Edbrooke L, et al. Deterioration in physical activity and function differs according to treatment type in non-small cell lung cancer - future directions for physiotherapy management. Physiotherapy 2016;102:256-63. [Crossref] [PubMed]
- Jones LW, Eves N, Haykowsky M, et al. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol 2009;10:598-605. [Crossref] [PubMed]
- Jones LW, Eves ND, Kraus WE, et al. The lung cancer exercise training study: a randomized trial of aerobic training, resistance training, or both in postsurgical lung cancer patients: rationale and design. BMC Cancer 2010;10:155. [Crossref] [PubMed]
- Jones LW, Eves N, Haykowsky M, et al. Cardiorespiratory exercise testing in clinical oncology research: systematic review and practice recommendations. Lancet Oncol 2008;9:757-65. [Crossref] [PubMed]
- Collins J, Noble S, Chester J, , et al. The assessment and impactof sarcopenia in lung cancer:a systematic literature review. BMJ Open 2014;4:e003697. [Crossref] [PubMed]
- Jones LW, Eves N, Kraus W, et al. The lung cancer exercise training study: a randomized trial of aerobic training, resistance training, or both in postsurgical lung cancer patients: rationale and design. BMC Cancer 2010;10:155. [Crossref] [PubMed]
- Maddocks M, Taylor V, Klezlova R, et al. When will I get my breath back? Recovery time of exercise-induced breathlessness in patients with thoracic cancer. Lung Cancer 2012;76:128-9. [Crossref] [PubMed]
- Burtin C, Franssen F, Vanfleteren L, et al. Lower-limb muscle function is a determinant of exercise tolerance after lung resection surgery in patients with lung cancer. Respirology 2017;22:1185-9. [Crossref] [PubMed]
- Sung MR, Patel MV, Djalalov S, et al. Evolution of Symptom Burden of Advanced Lung Cancer Over a Decade. Clin Lung Cancer 2017;18:274-80.e6. [Crossref] [PubMed]
- Koczywas M, Williams AC, Cristea M, et al. Longitudinal changes in function, symptom burden, and quality of life in patients with early-stage lung cancer. Ann Surg Oncol 2013;20:1788-97. [Crossref] [PubMed]
- Burtscher M. Exercise limitations by the oxygen delivery and utilizationsystems in aging and disease: coordinated adaptation and deadaptation of the lung-heart muscle axis - a mini-review. Gerontology 2013;59:289-96. [Crossref] [PubMed]
- Basch E, Deal AM, Kris MG, et al. Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J Clin Oncol 2016;34:557-65. [Crossref] [PubMed]
- Jung JY, Lee JM, Kim MS, et al. Comparison of fatigue, depression, and anxiety as factors affecting posttreatment health-related quality of life in lung cancer survivors. Psychooncology 2018;27:465-70. [Crossref] [PubMed]
- Chabowski M, Polanski J, Jankowska-Polanska B, et al. Is nutritional status associated with the level of anxiety, depression and pain in patients with lung cancer? J Thorac Dis 2018;10:2303-10. [Crossref] [PubMed]
- Chambers SK, Baade P, Youl P, et al. Psychological distress and quality of life in lung cancer: the role of health-related stigma, illness appraisals and social constraints. Psychooncology 2015;24:1569-77. [Crossref] [PubMed]
- Montazeri A, Milroy R, Hole D, et al. Anxiety and depression in patients with lung cancer before and after diagnosis: findings from a population in Glasgow, Scotland. J Epidemiol Community Health 1998;52:203-4. [Crossref] [PubMed]
- Chen HM, Tsai CM, Wu YC, et al. Randomised controlled trial on the effectiveness of home-based walking exercise on anxiety, depression and cancer-related symptoms in patients with lung cancer. Br J Cancer 2015;112:438-45. [Crossref] [PubMed]
- Dhillon HM, Bell ML, van der Ploeg HP, et al. Impact of physical activity on fatigue and quality of life in people with advanced lung cancer: A randomized controlled trial. Ann Oncol 2017;28:1889-97. [Crossref] [PubMed]
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Analysis of longitudinal quality-of-life data in high-risk operable patients with lung cancer: results from the ACOSOG Z4032 (Alliance) multicenter randomized trial. J Thorac Cardiovasc Surg 2015;149:718-25; discussion 725-6. [Crossref] [PubMed]
- Leach CR, Weaver KE, Aziz NM, et al. The complex health profile of long-term cancer survivors: prevalence and predictors of comorbid conditions. J Cancer Surviv 2015;9:239-51. [Crossref] [PubMed]
- Wang JW, Gong XH, Ding N, et al. The influence of comorbid chronic diseases and physical activity on quality of life in lung cancer survivors. Support Care Cancer 2015;23:1383-9. [Crossref] [PubMed]
- Bade BC, Thomas DD, Scott JB, et al. Increasing physical activity and exercise in lung cancer: reviewing safety, benefits, and application. J Thorac Oncol 2015;10:861-71. [Crossref] [PubMed]
- Cavalheri V, Granger C. Preoperative exercise training for patients with non-small cell lung cancer. Cochrane Database Syst Rev 2017;6:CD012020. [PubMed]
- Cavalheri V, Tahirah F, Nonoyama M, et al. Exercise training undertaken by people within 12 months of lung resection for non-small cell lung cancer. Cochrane Database Syst Rev 2013.CD009955. [PubMed]
- Sasso JP, Eves N, Christensen J, et al. A framework for prescription in exercise-oncology research. J Cachexia Sarcopenia Muscle 2015;6:115-24. [Crossref] [PubMed]
- Granger CL, Parry S, Edbrooke L, et al. Improving the delivery of physical activity services in lung cancer: a qualitative representation of the patient’s perspective. Eur J Cancer Care (Engl) 2018. [Epub ahead of print]. [Crossref] [PubMed]