
Predictors of blood transfusion and in-hospital outcomes in patients with gastric antral vascular ectasia (GAVE): a nationwide population-based analysis
Introduction
Gastric antral vascular ectasia (GAVE) is characterized by dilation of blood vessels in the antrum of the stomach. It is presented as long red lines in the stomach which appear like the markings on watermelon, therefore, it is also known as watermelon stomach (1). GAVE is one of the unusual causes of chronic upper gastrointestinal (GI) bleeding, representing around 4% of non-variceal upper GI bleeding. It usually presents as occult bleeding with chronic iron deficiency anemia (2). Even though GAVE was first diagnosed about four decades ago, its etiopathogenesis has not been fully established yet (3,4). Multiple published studies have mentioned various comorbidities such as chronic liver diseases, chronic kidney diseases, autoimmune diseases, connective tissue disorders and collagen vascular disorders associated with GAVE (5-8). However, all these published studies had a small number of GAVE patients, which might not be representative of the entire community. Although chronic GI bleeding is usually reported in the GAVE patients, it can also cause severe acute life-threatening bleeding especially in elderly with multiple chronic medical illnesses (2). Chronic blood transfusion dependent GAVE patients were also recognized which often requires multiple blood transfusions (9). Furthermore, the impact of GAVE on the nationwide healthcare resources utilization has never been evaluated. Therefore, the objective of our study was to recognize the demographics, hospitalization characteristics, comorbidities, hospitalization outcomes such as the inpatient mortality, length of stay (LOS), total hospital charges, discharge disposition and predictive factors of the packed red blood cell (PRBC) transfusion in hospitalized GAVE patients using the 2010–2014 National Inpatient Sample (NIS) database.
Methods
Data source
Our study cohort was queried from the NIS [2010–2014] dataset, which is developed by the Agency for Healthcare Research and Quality, as a part of Healthcare Cost and Utilization Project (HCUP). The NIS is the largest publicly accessible all-payer inpatient healthcare dataset in the United States (US). This database is considered as a stratified sample from 20% nonfederal community hospitals of the US. This dataset represents 95% of the US population. Each year dataset contains more than 7 million unweighted discharges which can be converted into weighted discharges (weight is calculated by the sum of discharges from all acute care hospitals in the US divided by the sum of discharges incorporated in the 20% sample) by the discharge weight (given in the dataset). More than 35 million weighted discharges per year were estimated in this dataset which represent national estimates. Up to 30 diagnoses (primary and secondary) and up to 15 procedures (primary and secondary) on each hospitalization were coded by International Classification of Diseases, 9th revision, Clinical Modification (ICD-9 CM). This de-identified dataset does not require authorization from the Institutional Review Board (IRB) to publish analysis from this database. This database comprises of various data elements such as the demographics (age, sex, day and type of the admission, race, median household income national quartile for patient zip code and primary expected payer), hospital characteristics (ownership/control, bed size, teaching status, urban/rural location, and geographic region), comorbidities measures, total hospital charges and LOS (10).
Study population
The ICD-9-CM codes 537.82 (GAVE without hemorrhage) and 537.83 (GAVE with hemorrhage) were utilized to recognize all hospitalized patients with a primary diagnosis of GAVE. Patients with a concomitant diagnosis of angiodysplasia of the intestine (ICD-9-CM code: 569.85 and 569.84) were omitted to avoid confounding. Then, a 1:2 random sample was attained from a non-GAVE population. Our study cohort comprised of 17,982 GAVE (weighted 89,081) and 36,000 non-GAVE (weighted 178,550) patients which were used for further analysis. Relevant comorbidities were recognized by using the validated ICD-9-CM codes (11) which were applied to the secondary diagnoses of GAVE patients.
Study outcomes
The primary outcomes of our study were to assess the baseline demographic, hospitalization characteristics and comorbidities measures associated with GAVE. Secondary outcomes were inpatients mortality, LOS, total hospitalization charges, disposition and requirement of PRBC transfusion in the hospitalized GAVE patients. Moreover, we also evaluated the predictors of PRBC transfusion in the GAVE cohort.
Statistical analysis
Pearson’s Chi-square test and Student t-test were utilized for assessing the categorical and continuous variables, correspondingly. The categorical and continuous variables were specified in percentages and mean ± SD, respectively. A two-tailed P value <0.05 was used to consider as statistical significant. Multivariate regression analysis was performed to assess the predicting factors associated with the PRBC transfusion in hospitalized GAVE patients after adjusting for the possible confounding factors such as the age, sex, race, admission day, type of admission, median household income according to the national quartile for patient zip code., payer status, hospitalization characteristics including bed size of hospital, region of hospital, control/ownership of hospital and location/teaching status of hospital. Multivariate logistic regression results were defined by the adjusted OR, 95% CI, and P value. SPSS version 22 (IBM Corp., Armonk, NY, USA) was used to execute all statistical analyses. We performed statistical analysis with weighted data to yield nationwide estimates.
Results
Baseline demographics and hospital characteristics
We included 17,982 GAVE (weighted 89,081) and 36,000 non-GAVE (weighted 178,550) patients in our study cohort. Table 1 displays the baseline demographics and hospitalization characteristics of the two study cohorts (non-GAVE vs. GAVE). The majority of the GAVE patients were 56–80 years old (48.4%), female (54.9%), White (70.2%) and Medicare enrollees (63.4%). Most GAVE patients had weekday (80.6%) and non-elective admissions (86.4%). The GAVE was more seen in the patients with lower income quartiles (29.2%). GAVE patients were more likely to be managed at large (62.8%), nonprofit private (76.9%), urban teaching hospital (49.2%) in southern (40.1%) part of the US. Compared to the non-GAVE group, GAVE patients were older (mean age in years 63.45±23.44 vs. 48.87±27.48, P<0.001), male (45.1% vs. 42.0%, P<0.001), White (70.2% vs. 65.8%, P<0.001) and African-American (15.6% vs. 15.1%, P<0.001).
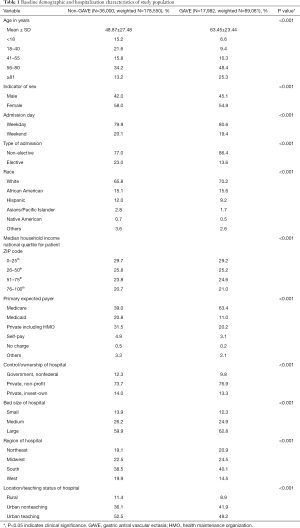
Full table
Comorbidity profile
Comorbidities such as any liver disease (9.3% vs. 2.4%, P<0.001), smoking (25.0% vs. 20.8%, P<0.001), alcohol abuse (4.8% vs. 4.0%, P<0.001), cirrhosis (2.2% vs. 0.7%, P<0.001), portal hypertension (3.9% vs. 0.5%, P<0.001), hypertension (59.9% vs. 41.5%, P<0.001), chronic pulmonary disease (24.9% vs. 15.3%, P<0.001), uncomplicated diabetes (24.9% vs. 15.7%, P<0.001), renal failure (23.3% vs. 9.8%, P<0.001), congestive heart failure (CHF) (20.1% vs. 7.1%, P<0.001), peripheral vascular disorder (10.8% vs. 4.7%, P<0.001), valvular heart disease (11.7% vs. 3.1%, P<0.001), diabetes with chronic complications (5.8% vs. 3.9%, P<0.001), hemodialysis (4.0% vs. 1.2%, P<0.001), solid tumor without metastasis (2.2% vs. 1.6%, P<0.001), Sjogren syndrome (0.2% vs. 0.1%, P<0.001), systemic sclerosis (0.4% vs. 0.1%, P<0.001), were higher in the GAVE cohort as compared to non-GAVE patients. However, comorbidities such as the rheumatoid arthritis/collagen vascular diseases (42.7% vs. 57.3%, P<0.001), drug abuse (2.0% vs. 3.6%, P<0.001), and obesity (9.5% vs. 10.0%, P<0.001) were lower in GAVE cohort as compared to non-GAVE cohort, whereas pernicious anemia (0.1% vs. 0.1%, P<0.001) and systemic lupus erythematosus (SLE) (0.5% vs. 0.5%, P=0.063) comorbidities were found equally in both cohorts (Table 2).
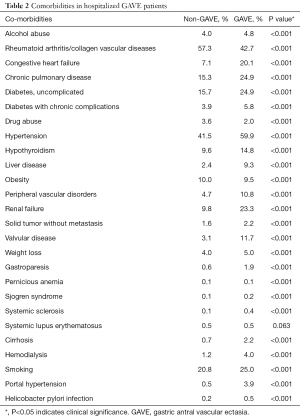
Full table
Hospitalization outcomes
Overall all-cause inpatient mortality in patients with GAVE was 1.4%. However, all-cause inpatient mortality was lower in the GAVE patients as compared to non-GAVE patients (1.4% vs. 1.8%, P<0.001). The mean total hospital charge and mean LOS (days) per GAVE-related hospitalization was $36,059 and 4.63±5.3 days, respectively. The mean total hospital charges ($36,929 vs. $36,059, P<0.001) and mean LOS (4.62±6.9 vs. 4.63±5.3, P<0.001) was almost the same in both cohorts. Deficiency anemias (22.5% vs. 14.7%, P<0.001), chronic blood loss anemia (15.8% vs. 2.1%, P<0.001) and iron deficiency anemia (6.0% vs. 2.1%, P<0.001) were found to be higher in the GAVE cohort as compared to non-GAVE patients. A total of 6,276 (weighted 31,102) (34.9%) of these patients received at least one PRBC transfusion during that hospitalization. As expected, GAVE patients were more likely to receive PRBC transfusion (34.9% vs. 5.2%, P<0.001) as compared to the non-GAVE patients. The GAVE patients were more likely to dispose to home health care (13.4% vs. 11.0%, P<0.001) and other transfers (skilled nursing facility (SNF), intermediate care facility (ICF), another facility) (14.7% vs. 13.6%, P<0.001) as compared to the non-GAVE patients (Table 3).
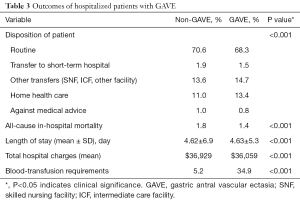
Full table
Predictors of PRBC transfusion in hospitalized GAVE patients.
Advanced age (OR =1.81, 95% CI: 1.77–1.85, P<0.001), male gender (OR =1.06, 95% CI: 1.03–1.10, P<0.001), African American (OR =1.18, 95% CI: 1.12–1.23, P<0.001) and Hispanic (OR =1.10, 95% CI: 1.03–1.17, P=0.002) race, higher household income (76–100th percentile; OR =1.09, 95% CI: 1.04–1.15, P<0.001), weekday (OR =1.11, 95% CI: 1.07–1.16, P<0.001) and non-elective admissions (OR =1.52, 95% CI: 1.44–1.60, P<0.001) and Medicare (OR =1.65, 95% CI: 1.45–1.88, P<0.001) and private including HMO (OR =1.44, 95% CI: 1.26–1.65, P<0.001) payer status was notably associated with an increase in the odds of PRBC transfusions in the GAVE patients. The odds of PRBC transfusions were higher in the patients hospitalized at the urban nonteaching (OR =1.21, 95% CI: 1.17–1.25, P<0.001), non-profit private (OR =1.22, 95% CI: 1.16–1.28, P<0.001), non-federal government (OR =1.18, 95% CI: 1.10–1.26, P<0.001) and Southern (OR =1.06, 95% CI: 1.01–1.11, P=0.024) region hospitals. Comorbidities like alcohol abuse (OR =1.15, 95% CI: 1.05–1.26, P=0.002), rheumatoid arthritis/collagen vascular disease (OR =1.12, 95% CI: 1.01–1.23, P=0.022), chronic pulmonary disease (OR =1.15, 95% CI: 1.11–1.20, P<0.001), diabetes (OR =1.11, 95% CI: 1.07–1.15, P<0.001), H. pylori infection (OR =1.93, 95% CI: 1.56–2.38, P<0.001), fluid and electrolytes disorders (OR =1.26, 95% CI: 1.21–1.30, P<0.001), peripheral vascular disorders (OR =1.14, 95% CI: 1.09–1.20, P<0.001), renal failure (OR =1.19, 95% CI: 1.14–1.24, P<0.001), solid tumor without metastasis (OR =1.16, 95% CI: 1.05–1.29, P=0.003), valvular disease (OR =1.39, 95% CI: 1.33–1.46, P<0.001), weight loss (OR =1.21, 95% CI: 1.13–1.30, P<0.001), systemic sclerosis (OR =1.78, 95% CI: 1.40–2.26, P<0.001), hemodialysis (OR =1.79, 95% CI: 1.65–1.95, P<0.001), liver disease (OR =1.75, 95% CI: 1.63–1.88, P<0.001), coagulopathy (OR =1.75, 95% CI: 1.65–1.85, P<0.001), smoking (OR =1.08, 95% CI: 1.04–1.12, P<0.001), portal hypertension (OR =1.44, 95% CI: 1.30–1.59, P<0.001) and CHF (OR =1.40, 95% CI: 1.34–1.46, P<0.001) were key independent predictors of PRBC transfusion in GAVE patients (Table 4).
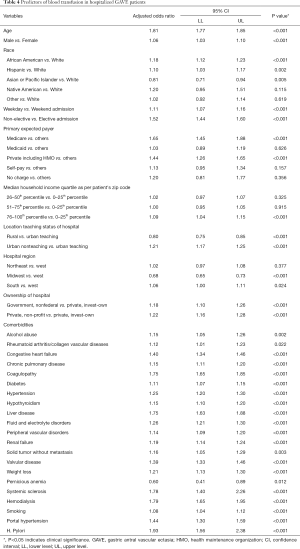
Full table
Discussion
We assessed the patients’ demographical characteristics, comorbidities, hospitalization outcomes and predictors associated with PRBC transfusion in GAVE hospitalizations from the largest nationwide in-hospital dataset [2010–2014] of the United States. The noteworthy results of this study are as follows: (I) GAVE patients were more likely to be older, female and white. Comorbidities such as the CHF, chronic pulmonary disease, diabetes, hypertension, hypothyroidism, liver disease, peripheral vascular disorder, renal failure, solid tumor without metastasis, valvular disease, weight loss, Sjogren syndrome, systemic sclerosis and portal hypertension were associated with hospitalized GAVE patients. (II) The overall all-cause inpatient mortality associated with GAVE patients was 1.4%. The mean total hospital charges and mean LOS (days) per GAVE hospitalization was $36,059 and 4.63±5.3 (days), respectively. (III) GAVE patients were more likely to be PRBC transfused and were more often transferred to other healthcare facilities as compared to the non-GAVE patients. (IV) Advanced age, male gender, African American race, Medicare payer type, non-elective admissions, higher household income group, and various comorbidities were independent factors associated with PRBC transfusion in GAVE cohort.
As we reported that patients with a primary diagnosis of GAVE were older, female and white, described similarly in other study cohorts too (7,12). However, the sample size of our study is larger as compared to the previously published studies. Therefore, the results of our study are more generalizable to the US population. In our study, various chronic medical problems such as hypertension, diabetes, chronic pulmonary disease, CHF, liver disease, renal diseases, etc. were reported in GAVE patients. It has been established that even though GAVE is not a common cause of upper GI bleeding, it can cause severe GI bleeding in the older patients who have multiple chronic illnesses (2). Therefore, it is vital to rule out the GAVE in elderly patients especially with cardiac, pulmonary, hepatic and renal-related chronic diseases, who are diagnosed with the occult bleeding or severe upper GI bleeding with severe anemia (13). GAVE has also been reported to be commonly present in patients with various autoimmune disorders which could be one of its etiologies (14,15). We also revealed that the autoimmune disorders such as systemic sclerosis and Sjogren syndrome were also more confined to GAVE cohort. However, few autoimmune disorders such as the SLE and pernicious anemia were found equally in both GAVE and non-GAVE cohorts. Moreover, the results of our study revealed that rheumatoid arthritis/collagen vascular diseases tended to be lower in the GAVE cohort as compared to the non-GAVE cohort. In autoimmune and connective tissue diseases, mechanical stress, dysfunction of antropyloric motility and disordered peristaltic waves can prolapse of gastric mucosa in the distal part of the stomach which can lead to the development of ectatic blood vessels (16,17). Many cases of GAVE were reported with end-stage renal disease (18-20). Similarly, we found renal failure (23.3%) was highly associated with GAVE cohorts too. Zuckerman et al. reported that nearly 50% of recurrent GI bleeding in renal failure patients was due to angiodysplasia of the stomach and duodenum (21). In renal diseases, uremia can cause weakening of gastric emptiness which may increase mechanical stress on antrum which could be the reason for the development of GAVE. Impaired renal excretion and/or catabolism of vasoactive hormones like gastrin and prostaglandin E2 might be an explanation for the development of GAVE (22). It is accounted that nearly 30% GAVE patients were associated with cirrhosis (23). However, we found that only 9.3%, 2.2%, and 3.9% of patients were associated with liver disease, cirrhosis and portal hypertension in our study cohorts. Increased levels of gastrin and prostaglandin E2 which has vasodilating properties were noted in GAVE patients. These hormones may build up in liver disease because it cannot be processed efficiently, explained to the pathogenesis of GAVE (24).
It is established that most GAVE patients present with iron deficiency anemia owing to chronic blood loss (7). In that manner, we reported 22.5%, 15.8%, and 6.0% GAVE patients were diagnosed with deficiency anemias, chronic blood loss anemia, and iron deficiency anemia, respectively. There is a lack of data on the requirement of PRBC transfusion in the hospitalized GAVE patients. We observed that more than one-third of (34.9%) GAVE patients received at least one PRBC transfusions in our study cohort. Our study reported 1.4% all-cause inpatient mortality in the hospitalized GAVE patients which was found to be lower as compared to the non-GAVE cohort (1.4% vs. 1.8%). It could be assumed to be due to the fewer complications related to GAVE and availability of various treatment modalities such as proton pump inhibitors, endoscopy, and surgery to treat GAVE (25). However, Kar et al. reported the higher morbidity and mortality in GAVE patients with underlying chronic medical illness (23). The mean total hospital charges and mean LOS (days) per GAVE hospitalization was $36,059 and 4.63±5.3 (days), respectively. However, there were no differences in the mean total hospital charges and mean LOS between the two cohorts.
Notably, the predicting factors associated with the PRBC transfusions in the hospitalized GAVE patients were advanced age, male gender, African American and Hispanic race, weekday and non-elective admissions, Medicare and private insurance including HMO payer type and higher household income. These factors have not been explored in the previous studies. Another unique finding of our study was that the hospitalized GAVE patients who were admitted to a non-profit private, non-federal government, urban non-teaching and Southern hospitals were more likely to get PRBC transfusions. Cardiovascular risk factors and diseases such as hypertension, diabetes, smoking, CHF and valvular diseases increased the risk of PRBC transfusions in the GAVE patients. Other chronic illnesses such as chronic pulmonary disease, liver disease, PVD, renal failure, hypothyroidism, alcohol abuse, coagulopathy, solid tumor without metastasis and portal hypertension raised the odds of PRBC transfusions in GAVE cohort. Autoimmune diseases such as systemic sclerosis and rheumatoid arthritis/collagen vascular disease were also significant predictors of PRBC transfusions in the GAVE cohort. Hospitalized GAVE patients who were on hemodialysis and were infected with H. pylori had an increased chance of PRBC transfusion.
A few limitations cannot be overlooked in this study. The ICD-9-CM coding errors can be possible in this administrative dataset. We could not study the endoscopic finding of GAVE patients due to the unavailability of the data. Thus, over or underestimation of GAVE is possible in the minority of instances. As mentioned earlier, we included only hospitalized GAVE patients, therefore, we could not apply these results to GAVE patients who do not necessitate hospitalization. We were not able to delineate the frequency of PRBC transfusion in GAVE patients owing to the nature of data. Regardless of limitations, this is the first and the largest nationwide study that explored the outcomes of GAVE hospitalization and predictive factors of PRBC transfusion in the GAVE patients.
Conclusions
Our study concluded that the hospitalized GAVE patients were more likely to be older, female and white. GAVE cohort had more frequent cardiovascular risk factors and ailments, chronic medical illnesses, autoimmune diseases and different types of anemias as compared to non-GAVE cohorts. The overall all-cause inpatient mortality associated with GAVE patients was 1.4%. There were no statistical differences in the mean LOS (days) and total hospital charges. Nearly 1/3rd of the GAVE patients received at least one PRBC transfusion during their hospitalization. Advanced age, male gender, African American race, Medicare payer type, non-elective admissions, higher household income group and various comorbidities were independent factors associated with the PRBC transfusion in GAVE cohort. A systemic and comprehensive approach should be considered for identifying and managing factors associated with the blood transfusion in GAVE patients to reduce the utilization of health care resources and improve the clinical outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This de-identified dataset does not require authorization from the Institutional Review Board (IRB) to publish analysis from this database.
References
- Suit PF, Petras RE, Bauer TW, et al. Gastric antral vascular ectasia. A histologic and morphometric study of "the watermelon stomach". Am J Surg Pathol 1987;11:750-7. [Crossref] [PubMed]
- Alkhormi AM, Memon MY, Alqarawi A. Gastric Antral Vascular Ectasia: A Case Report and Literature Review. J Transl Int Med 2018;6:47-51. [Crossref] [PubMed]
- Yildiz B, Sokmensuer C, Kaynaroglu V. Chronic anemia due to watermelon stomach. Ann Saudi Med 2010;30:156-8. [Crossref] [PubMed]
- Tuveri M, Borsezio V, Gabbas A, et al. Gastric antral vascular ectasia--an unusual cause of gastric outlet obstruction: report of a case. Surg Today 2007;37:503-5. [Crossref] [PubMed]
- Spahr L, Villeneuve JP, Dufresne MP, et al. Gastric antral vascular ectasia in cirrhotic patients: absence of relation with portal hypertension. Gut 1999;44:739-42. [Crossref] [PubMed]
- Calamia KT, Scolapio JS, Viggiano TR. Endoscopic YAG laser treatment of watermelon stomach (gastric antral vascular ectasia) in patients with systemic sclerosis. Clin Exp Rheumatol 2000;18:605-8. [PubMed]
- Gostout CJ, Viggiano TR, Ahlquist DA, et al. The clinical and endoscopic spectrum of the watermelon stomach. J Clin Gastroenterol 1992;15:256-63. [Crossref] [PubMed]
- Montagnac R, Blaison D, Brahimi S, et al. Watermelon stomach: Chronic renal failure and/or imatinib? Nephrol Ther 2015;11:496-501. [Crossref] [PubMed]
- Dunne KA, Hill J, Dillon JF. Treatment of chronic transfusion-dependent gastric antral vascular ectasia (watermelon stomach) with thalidomide. Eur J Gastroenterol Hepatol 2006;18:455-6. [Crossref] [PubMed]
- HCUP Databases. Healthcare Cost and Utilization Project (HCUP). August 2018. Agency for Healthcare Research and Quality, Rockville, MD. Available online: www.hcup-us.ahrq.gov/nisoverview.jsp
- Patel SD, Desai R, Patel U, et al. Thirty-day Readmissions After Upper and Lower Gastrointestinal Hemorrhage: A National Perspective in the United States. J Clin Gastroenterol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Ingraham KM, O'Brien MS, Shenin M, et al. Gastric antral vascular ectasia in systemic sclerosis: demographics and disease predictors. J Rheumatol 2010;37:603-7. [Crossref] [PubMed]
- Nguyen H, Le C, Nguyen H. Gastric antral vascular ectasia (watermelon stomach)-an enigmatic and often-overlooked cause of gastrointestinal bleeding in the elderly. Perm J 2009;13:46-9. [Crossref] [PubMed]
- Beales IL. Watermelon stomach in the CREST syndrome. Postgrad Med J 1994;70:766-7. [Crossref] [PubMed]
- Krstić M, Alempijevic T, Andrejevic S, et al. Watermelon stomach in a patient with primary Sjogren's syndrome. Vojnosanit Pregl 2010;67:256-8. [Crossref] [PubMed]
- Charneau J, Petit R, Cales P, et al. Antral motility in patients with cirrhosis with or without gastric antral vascular ectasia. Gut 1995;37:488-92. [Crossref] [PubMed]
- Quintero E, Pique JM, Bombi JA, et al. Gastric mucosal vascular ectasias causing bleeding in cirrhosis. A distinct entity associated with hypergastrinemia and low serum levels of pepsinogen I. Gastroenterology 1987;93:1054-61. [Crossref] [PubMed]
- Pisharam JK, Ramaswami A, Chong VH, et al. Watermelon stomach: a rare cause of anemia in patients with end-stage renal disease. Clin Nephrol 2014;81:58-62. [Crossref] [PubMed]
- Lata S, Gupta V, Nandwani A, et al. Watermelon stomach in end-stage renal disease patient. Indian J Nephrol 2012;22:477-9. [Crossref] [PubMed]
- Lin WH, Cheng MF, Cheng HC, et al. Watermelon stomach in a uremia patient. Kidney Int 2010;78:821. [Crossref] [PubMed]
- Zuckerman GR, Cornette GL, Clouse RE, et al. Upper gastrointestinal bleeding in patients with chronic renal failure. Ann Intern Med 1985;102:588-92. [Crossref] [PubMed]
- Iguchi A, James Kazama J, Komatsu M, et al. Three cases of gastric antral vascular ectasia in chronic renal failure. Case Rep Nephrol Urol 2011;1:15-9. [Crossref] [PubMed]
- Kar P, Mitra S, Resnick JM, et al. Gastric Antral Vascular Ectasia: Case Report and Review of the Literature. Clinical Medicine & Research 2013;11:80-5. [Crossref] [PubMed]
- Ripoll C, Garcia-Tsao G. The management of portal hypertensive gastropathy and gastric antral vascular ectasia. Dig Liver Dis 2011;43:345-51. [Crossref] [PubMed]
- Fuccio L, Mussetto A, Laterza L, et al. Diagnosis and management of gastric antral vascular ectasia. World J Gastrointest Endosc 2013;5:6-13. [Crossref] [PubMed]


