
Prediction of pleural invasion using different imaging tools in non-small cell lung cancer
Introduction
Clinical staging of non-small cell lung cancer (NSCLC) is used for guiding therapeutic planning for the patient; therefore, accurate diagnosis and staging in the preoperative period is important. Several studies (1,2) have shown that visceral pleural invasion (VPI) is a poor prognostic factor in early stage NSCLC. According to these previous reports, pleural invasion of NSCLC has been adopted as a T impact factor in the TMN staging system of UICC since 1970s (3,4). Based on the 7th edition TNM staging of lung cancer, pleural invasion has been designated as PL0 (tumor does not invade beyond the elastic layer), PL1 (tumor invades beyond the elastic layer), PL2 (tumor invades the visceral pleural surface), and PL3 (tumor invades the parietal pleural) (5). PL0 is not defined as VPI. The TNM staging system defined PL1 and PL2 as VPI (staged as T2) and PL3 as parietal pleural invasion (staged as T3). Further, the latest 8th TNM staging has retained the same pleural invasion classification (6) (Figures 1,2).
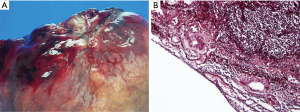
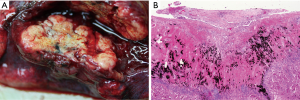
The revised staging of NSCLC has a strong impact on the treatment strategies. For example, extrapleural dissection with en-bloc resection were recommended for parietal pleural invasion (7), and lobectomy instead of segmentectomy was recommended in patients with VPI (8). Therefore, noninvasiveness of early lung cancer should be precisely identified in preoperative radiological evaluations, as limited sublobar resection surgery (segmentectomy or wedge resection) is indicated in such cases.
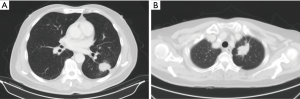
Chest radiography (CXR) is the most common tool for the initial investigation of lung disease. However, CXR is not suitable for assessing pleural invasion in early stage NSCLC. Ultrasound (US) (9,10), computed tomography (CT) (11-21), magnetic resonance imaging (MRI) (22,23) and fluorine-18-fluorodeoxyglucose positron emission tomography (18F-FDG PET) (24-26) have been used for preoperative prediction of pleural invasion. However, no single imaging method has been proposed for the definitive diagnosis of pleural invasion, and each imaging modality has its advantages as well as limitations (Figure 3). These imaging tools have the potential to facilitate better clinical decision-making, particularly in the treatment of patients with cancer (27). Therefore, to identify the appropriate imaging modality for this purpose, we aimed to review the imaging methods indicated in published reports for predicting pleural invasion of NSCLC.
Ultrasonography
US is a non-invasive, widely accessible, and low-cost tool used for medical imaging. It has been used for evaluation of chest wall invasion (PL3) (9). If the mass is observed to be attached to the pleura, with loss of movement during respiration under US examination, chest wall invasion should be highly suspected. A prospective study that included 90 patients revealed that, compared to CT scan examination (sensitivity, 42%; specificity, 100%) (10), US has better sensitivity (89%) and specificity (95%) for assessing chest wall involvement by a lung tumor.
Preoperative prediction of pleural invasion by US has some limitations. First, the accuracy of intraoperative US is highly dependent on the skill and experience of the operator. Second, it is difficult to predict VPI (PL1 and PL2) by using US; this is because VPI does not involve the chest wall and does not affect pleural movement during respiration. Currently, reports on predicting VPI using US are lacking.
CT
Prediction of chest wall invasion
Chest CT and bone scan are usually used for preoperative evaluation of the depth of chest wall invasion. According to pathological findings, invasion of only the parietal pleura is defined as shallow invasion, and invasion of the soft tissue or ribs is defined as deep invasion.
Kawaguchi et al. (11) retrospectively surveyed 132 patients who had undergone resection for NSCLC involving the chest wall, and they showed that tumor invasion beyond the parietal pleura on the preoperative CT [hazard ratio (HR) =6.824, P=0.005] and complaints of chest pain (HR =3.282, P=0.015) were independent indicators of deep invasion into the chest wall. En-bloc resection was recommended in patients with chest pain and/or deep invasion on chest CT.
Tumor disappearance ratio (TDR)
The pure ground grass opacity component of the lung on CT is typically believed to indicate non-invasive or precancerous lesions. In contrast, the solid component has been recognized to indicate invasive lesions. Evaluation of the TDR is one of the methods used to evaluate the proportion of tumor in order to predict tumor invasiveness (12-14). TDR is defined as 1-DM/DL (DM: maximum tumor dimension on mediastinal window, DL: maximum tumor dimension on lung window). Tumor size is evaluated using both the mediastinal window setting and lung window setting; evaluation of tumor size using this setting is considered to have greater prognostic value than that that only using the lung window setting.
Some studies used TDR to predict pathologically noninvasive (without vessel invasion, pleural invasion, and lymph node metastasis) NSCLC. Shimada et al. (12) analyzed 363 patients and showed that the combination of a TDR larger than 0.5 and the absence of spiculation was highly predictive of noninvasive or minimally invasive T1aN0M0 peripheral NSCLC (P<0.001). However, TDR may be used to indicate only tumor invasiveness; it could not predict pleural invasion precisely.
Consolidation-to-tumor ratio (CTR)
CTR is another method for analyzing whether radiologic density of tumor could be regarded as a predictor of tumor aggressiveness. CTR is defined as the ratio of the maximum consolidation diameter (C) to maximum tumor diameter (T) on lung window (15-17). It has been used to predict the risk of pleural invasion indirectly by predicting the extent of tumor aggressiveness.
Suzuki et al. (15) reported that CTR on lung window [window level = −500 to −700 Hounsfield unit (HU), window width =1,000 to 2,000 HU] could be used to predict pathologically noninvasive (without nodal involvement, vascular invasion, or lymphatic invasion) lung cancer, and CTR less than 0.25 was considered to indicate radiological noninvasiveness in T1N0M0 (tumor size less than 2 cm) NSCLC (sensitivity: 16.2%, specificity: 98.7%). Similar to TDR, CTR may also be indicated for predicting only tumor invasiveness; it could not predict pleural invasion precisely.
Tumor border analysis
Although CT is widely used for staging NSCLC, its ability to predict pleural invasion is still limited. A tumor abutting the pleural surface does not indicate invasion. Many methods for predicting pleural invasion using CT have been published (18-21), one of which is analysis of the tumor border.
Some radiologists diagnosed pleural invasion of NSCLC by using conventional CT criteria combined with at least two of the following three criteria: tumor size more than 3 cm, with contact with the neighboring pleura; obtuse angle with the contact surface; and related pleural thickening. Two expert radiologists using conventional criteria showed that the sensitivity and specificity rates were 46.7% and 74.2%, and 91.3% and 84.8%, respectively (18).
Another method used to predict pleural invasion was the measurement of the ratio of the arch distance (interface between the tumor and surrounding structures) to the maximum diameter of the tumor (19). For this ratio, a value more than 0.9 indicated an accurate diagnosis of chest wall invasion (PL3) (sensitivity and specificity for thoracic invasion, 89.7% and 96.0%).
In addition, pleural tags were also mentioned and were defined as one or more linear strands that extend from the tumor surface to the pleural surface. A retrospective study (20) classified pleural tags on CT into 3 types (type 1, one or more linear pleural tag; type 2, one or more linear pleural tag with a soft tissue component at the pleural end; and type 3, one or more soft tissue cord-like pleural tag); type 2 pleural tag has the statistical significance on the prediction of the VPI by NSCLC that is not abutting the pleura [positive likelihood ratio (LR): 5.06; accuracy: 71%; sensitivity: 36.4%; specificity: 92.8%, P<0.001].
Hsu et al. (21) evaluated the tumor border for predicting plural invasion in peripheral NSCLC in 136 patients. A retrospective study was performed and the tumor border was classified into 5 types. The type 5 tumor border (convex border with perpendicular or blunt angle) has a high positive predictive value (PPV) and specificity for predicting pleural invasion of peripheral NSCLC (LR: 5.20; accuracy; 57%; sensitivity: 45%; specificity: 91%; PPV: 94%; and negative predictive value: 36%). They also reported that a blunt angle, pleural contact >3 cm, and pleural thickening are not significantly different in patients with and without pleural invasion.
MRI
The basic principle of using MRI to evaluate pleural invasion is similar to that for the use of US. Physicians may use respiratory dynamic MRI to assess the movement of a tumor abutting the chest wall during breathing; tumors without parietal pleura invasion are expected to show movement with each breath. Akata et al. (22) reported that respiratory dynamic MRI improved the accuracy for predicting chest wall invasion of NSCLC (sensitivity: 100%, specificity: 82.9%).
Dynamic MRI is a dynamic evaluation, similar to US, for chest wall invasion. However, these dynamic evaluations could not distinguish between invasion and adhesion, and it should be exclusively used for the prediction of parietal pleura invasion or chest wall invasion. Another similar and practical method for evaluation of parietal pleura invasion is the dynamic CT scan. The advanced technology makes CT scans much faster and more precise than before. It can provide precise information on the movement of a tumor and nearby organs. The speed and spatial resolution of the 320-slice CT make it a useful and non-invasive tool for the initial evaluation of cardiac disease. Choong et al. (23) tried to use 320-slice CT for detecting invasion of lung tumors into the adjacent structures and termed as dynamic four-dimensional CT (4D CT); eight cases of NSCLC were evaluated. They revealed that this modality may be useful in the preoperative assessment of invasion into adjacent structures. However, few reports have been published on the use of 4D CT for pleural invasion.
PET
PET is an important tool for staging of cancer. It is usually used to assess metastasis and localize mediastinal nodal involvement (24,25) in patients with suspected cancer. However, it is difficult to assess pleural invasion by PET directly. Generally, 18F-FDG uptake is highly correlated with tumor invasiveness. The higher is the 18F-FDG uptake, the higher are the proliferation and invasion exhibited by tumors. Therefore, 18F-FDG PET/CT was used for predicting VPI according to the SUVmax. Tanaka et al. (26) used SUVmax 4.3 on 18F-FDG PET/CT as the cutoff value for predicting VPI in lung adenocarcinoma with pleural contact. In their study, 208 patients were analyzed, and 85% accuracy was observed. In contrast, no case with SUVmax <1.3 showed pleural invasion (any tumor size). Therefore, pleural contact with high SUV may be a predictive factor for pathological VPI in lung adenocarcinoma.
Conclusions
Among CXR, US, PET scan, CT scan, and MRI, CXR remains the modality of choice for the initial investigation of patients with suspected pleural disease, such as mesothelioma or pleural effusion. US is useful for monitoring pleural changes, but the accuracy of US for detecting VPI in NSCLC is not advocated. PET and PET/CT is useful for the differentiation of malignant and benign pleural lesions and effusions. Further, CT scan is best understood by appreciating the different morphology in pleura, especially in patients with small tumor size.
In future, we believe there will be more technical challenges in the process of radiomic analysis, and will be more clinical applications for radiomic analysis in lung cancer image researches (27-29). These novel techniques may provide more precise information for preoperative prediction of pleural invasion and may improve the diagnostic accuracy and clinical outcomes in patients with lung cancer.
Acknowledgements
Funding: This study was funded by the National Taiwan University Hospital, Taipei, Taiwan (NTUH107-N004038, MS-419), and the Ministry of Science and Technology, Taiwan (MOST 107-2221-E-002-080-MY3).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Huang H, Wang T, Hu B, et al. Visceral pleural invasion remains a size-independent prognostic factor in stage I non-small cell lung cancer. Ann Thorac Surg 2015;99:1130-9. [Crossref] [PubMed]
- Kudo Y, Saji H, Shimada Y, et al. Impact of visceral pleural invasion on the survival of patients with non-small cell lung cancer. Lung Cancer 2012;78:153-60. [Crossref] [PubMed]
- Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710-7. [Crossref] [PubMed]
- Ichinose Y, Yano T, Asoh H, et al. Prognostic factors obtained by a pathologic examination in completely resected non-small-cell lung cancer. An analysis in each pathologic stage. J Thorac Cardiovasc Surg 1995;110:601-5. [Crossref] [PubMed]
- Rami-Porta R, Ball D, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2007;2:593-602. [Crossref] [PubMed]
- Rami-Porta R, Asamura H, Travis WD, et al. Lung cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:138-55.
- Santos HT, Lopes AJ, Higa C, et al. Lung cancer with chest wall invasion: retrospective analysis comparing en-bloc resection and ‘resection in bird cage’. J Cardiothorac Surg 2014;9:57. [Crossref] [PubMed]
- Jeon HW, Kim YD, Kim KS, et al. Sublobar resection versus lobectomy in solid-type, clinical stage IA, non-small cell lung cancer. World J Surg Oncol 2014;12:215. [Crossref] [PubMed]
- Sugama Y, Tamaki S, Kitamura S, et al. Ultrasonographic evaluation of pleural and chest wall invasion of lung cancer. Chest 1988;93:275-9. [Crossref] [PubMed]
- Bandi V, Lunn W, Ernst A, et al. Ultrasound vs. CT in detecting chest wall invasion by tumor: a prospective study. Chest 2008;133:881-6. [Crossref] [PubMed]
- Kawaguchi K, Mori S, Usami N, et al. Preoperative evaluation of the depth of chest wall invasion and the extent of combined resections in lung cancer patients. Lung Cancer 2009;64:41-4. [Crossref] [PubMed]
- Shimada Y, Yoshida J, Hishida T, et al. Predictive factors of pathologically proven noninvasive tumor characteristics in T1aN0M0 peripheral non-small cell lung cancer. Chest 2012;141:1003-9. [Crossref] [PubMed]
- Ito T, Murakawa T, Sato H, et al. Simple preoperative computed tomography image analysis shows good predictive performance for pathological vessel invasion in clinical stage IA non-small cell lung cancer. Interact Cardiovasc Thorac Surg 2012;15:633-8. [Crossref] [PubMed]
- Sakao Y, Kuroda H, Mun M, et al. Prognostic significance of tumor size of small lung adenocarcinomas evaluated with mediastinal window settings on computed tomography. PLoS One 2014;9:e110305. [Crossref] [PubMed]
- Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011;6:751-6. [Crossref] [PubMed]
- Asamura H, Hishida T, Suzuki K, et al. Radiographically determined noninvasive adenocarcinoma of the lung: survival outcomes of Japan Clinical Oncology Group 0201. J Thorac Cardiovasc Surg 2013;146:24-30. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Quint LE, Francis IR, Wahl RL, et al. Preoperative staging of non-small-cell carcinoma of the lung: imaging methods. AJR Am J Roentgenol 1995;164:1349-59. [Crossref] [PubMed]
- Imai K, Minamiya Y, Ishiyama K, et al. Use of CT to evaluate pleural invasion in non-small cell lung cancer: measurement of the ratio of the interface between tumor and neighboring structures to maximum tumor diameter. Radiology 2013;267:619-26. [Crossref] [PubMed]
- Hsu JS, Han IT, Tsai TH, et al. Pleural Tags on CT Scans to Predict Visceral Pleural Invasion of Non-Small Cell Lung Cancer That Does Not Abut the Pleura. Radiology 2016;279:590-6. [Crossref] [PubMed]
- Hsu JS, Jaw TS, Yang CJ, et al. Convex border of peripheral non-small cell lung cancer on CT images as a potential indicator of pleural invasion. Medicine (Baltimore) 2017;96:e7323. [Crossref] [PubMed]
- Akata S, Kajiwara N, Park J, et al. Evaluation of chest wall invasion by lung cancer using respiratory dynamic MRI. J Med Imaging Radiat Oncol 2008;52:36-9. [Crossref] [PubMed]
- Choong CK, Pasricha SS, Li X, et al. Dynamic four-dimensional computed tomography for preoperative assessment of lung cancer invasion into adjacent structuresdagger. Eur J Cardiothorac Surg 2015;47:239-43; discussion 243. [Crossref] [PubMed]
- Vansteenkiste JF. Imaging in lung cancer: positron emission tomography scan. Eur Respir J Suppl 2002;35:49-60s. [Crossref] [PubMed]
- Higashi K, Ueda Y, Arisaka Y, et al. 18F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med 2002;43:39-45. [PubMed]
- Tanaka T, Shinya T, Sato S, et al. Predicting pleural invasion using HRCT and 18F-FDG PET/CT in lung adenocarcinoma with pleural contact. Ann Nucl Med 2015;29:757-65. [Crossref] [PubMed]
- Yang SM, Chen LW, Wang HJ, et al. Extraction of radiomic values from lung adenocarcinoma with near-pure subtypes in the International Association for the Study of Lung Cancer/the American Thoracic Society/the European Respiratory Society (IASLC/ATS/ERS) classification. Lung Cancer 2018;119:56-63. [PubMed]
- Hsieh MS, Lee YH, Lin MW, et al. Solitary pulmonary capillary hemangioma: An under-recognized pulmonary lesion mimicking early lung cancer on computed tomography images. Lung Cancer 2018;124:227-32. [Crossref] [PubMed]
- Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016;278:563-77. [Crossref] [PubMed]


