
Role of bronchoalveolar lavage in the management of immunocompromised patients with pulmonary infiltrates
Introduction
Pulmonary infiltrates are a significant cause of morbidity and mortality in immunocompromised patients (1-3). A mortality rate as high as 77% has been reported along with severe morbidity including 54% requiring admission to the intensive care unit (ICU) and 46% requiring mechanical ventilation (3). These patients are diagnostic challenges due to their vulnerability to an array of conditions including opportunistic infection and malignancy (1).
Establishing a confirmed diagnosis early in the course of disease has been associated with reduced mortality (32% vs. 51%, P=0.024) and provides important information to guide therapy (3). Empiric antibiotics is also associated with risk of ineffective therapy and development of antimicrobial resistance. Flexible bronchoscopy with bronchoalveolar lavage (BAL) may be performed in an ambulatory setting with moderate sedation and is often used for investigating pulmonary infiltrates (4). Meta-analysis data of randomized controlled trials studying sedation in bronchoscopy found that moderate sedation with either benzodiazepines or propofol improved acceptability of bronchoscopy (5). Patients who received sedation demonstrated significantly greater willingness to undergo a repeat bronchoscopy [odds ratio (OR) 2.30, 95% CI: 1.11–4.73, P=0.02, I2=22.5%] (5). In addition, duration of bronchoscopy was found to be significantly shorter for patients under sedation [standardized mean difference (SMD) −0.21; 95% CI: −0.38 to −0.03, P=0.02, I2=78.3%] (5). However, there are potential risks associated with moderate sedation including hemodynamic and respiratory compromise.
The process of BAL involves advancing the flexible bronchoscope towards the pulmonary segment corresponding to the location of infiltrates on computed tomography scan or towards the right middle lobe or lingular segment for diffuse lung disease (4,6). The bronchoscope is wedged at the airway orifice and 100 to 300 mL of sterile saline is instilled in 3–5 divided aliquots before being gently suctioned out for analysis (6). Ideally >30% of instilled volume should be retrieved for diagnostic studies that include cytologic studies and microbiologic analysis (6). BAL samples secretions from smaller airways and differs from bronchial washing that obtain samples from larger airways and do not necessarily sample secretions from the lung parenchyma. In bronchial washing, the scope is not wedged into position during collection of instilled saline. Although bronchial washing and BAL may be comparable in obtaining a microbiological diagnosis, BAL diagnostic yield is more commonly reported in the published literature and there is insufficient data to compare the two procedures. Both procedures carry risk of complications such as hypoxemia.
The immunocompromised patient represents a heterogeneous group of patients with a range of etiology of underlying immunosuppression from congenital causes to acquired causes such as human immunodeficiency virus (HIV) infection and drug-induced causes from chemotherapy or immunosuppressants. The Infectious Diseases Society of America 2013 guidelines has provided clear definitions of high-grade immunosuppression (Table 1) (7). In addition to those specified in Table 1, common etiologies of immunocompromise include ongoing hematologic malignancies, myeloproliferative disorders and steroid-sparing immunosuppressants (8,9). Without a consensus on what constitutes the immunosuppressed patient, making comparisons on BAL diagnostic yield becomes challenging.
Despite the differences in underlying etiology, immunocompromised patients with pulmonary infiltrates present with similar clinical symptoms of cough, fever and dyspnoea. They often require urgent confirmation of diagnosis to guide management and are also physiologically fragile with a guarded prognosis. In addition, BAL is not without risks. The current literature does not provide clear answers as to who will benefit from such invasive diagnostic testing, i.e., no ideal patient selection to maximize diagnostic efficacy and safety. To identify gaps where research is needed, this review has analyzed the current published literature on the diagnostic yield and safety profile of BAL in immunocompromised patients with pulmonary infiltrates.
Methods
A search of the literature was performed using PubMed from January 2000 to June 2018. The following search terms were used: “immunocompromised host”, “immunocompromise”, “immunosuppression”, “bronchoalveolar lavage” and “diagnostic yield”. Both prospective and retrospective studies involving bronchoalveolar lavage and immunocompromised host were included. Case reports and paediatric studies were excluded. Forest plot representation of data was not attempted because of heterogeneity of study design and wide variation in inclusion criteria that made generalization not valid. To meet inclusion into this review, studies needed to report diagnostic yield.
Studies that focused on patients in the ICU setting or mechanical ventilation were excluded due to differences in procedure and prognosis and the procedure by which bronchoscopy is performed on intubated patients. The risks of performing BAL when the patient is already intubated and sedated for mechanical ventilation are different from BAL performed in an ambulatory setting. Furthermore, patients with respiratory failure on mechanical ventilation have high mortality of up to 77% (71/92) and should be considered separately (3). These features add heterogeneity that further confounds any conclusions that can be made. Therefore, this review is focused on the diagnostic utility and safety of BAL in the ambulatory setting on immunocompromised patients who presented with pulmonary infiltrates. All identified studies were independently reviewed by both authors and only data that had consensus was included in the analysis. Diagnostic yield was defined as either a confirmed microbiological or cytological diagnosis that was compatible with the clinical presentation. Post-procedural treatment modification was reported as present when results of the BAL are used to guide treatment such as modification of antimicrobial coverage or initiating therapy for non-infectious causes (10).
Results
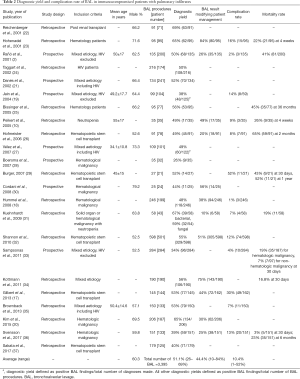
A total of 29 studies were identified through the PubMed search. One publication did not report diagnostic yield, two were ICU studies and three further studies had limited data on BAL (11-16). These 6 studies were excluded from our analysis. Two further studies included a minority of patients on mechanical ventilation, i.e., 14.5% (29/200) and 32.7% (53/162) (2,17). In addition, one other study included some ICU patients, i.e., 28.5% (71/249) (18). In four further studies, there was a possibility that some of patients were on mechanical ventilation, but exact numbers were unavailable from the publication (19-21). Despite the heterogeneity in inclusion criteria, these studies were analyzed in our review to provide the broadest evidence for the use of BAL to investigate pulmonary infiltrates in immunocompromised patients. Therefore, 23 studies were included in our review (Table 2).
Full table
There were seven prospective studies and 16 retrospective studies with 3,395 BAL procedures performed on 3,192 patients. The average diagnostic yield of BAL was 51.1%; range 26% to 69% (Table 2). The clinical impact of BAL as assessed by treatment modification based on BAL findings was reported in 11 studies with an average rate of modification being 44.4%, ranging from 11% to 84%. Overall mortality of immunocompromised patients presenting with pulmonary infiltrates was reported in 8 studies, but mortality was reported at different time points which made pooling of data unfeasible. One-month mortality ranged from 3% to 22% in hematologic malignancy (Table 2). In neutropenic patients 1-month mortality was 26% and 42.9% in those post hematopoietic stem cell transplant (Table 2). One study identified lower mortality if diagnosis was confirmed within 4 days of presentation while another study identified a higher mortality in patients with hematologic malignancies compared to other causes of immunocompromise (19% vs. 7%, P<0.05) (32,33).
Underlying etiology of immunosuppression
One study reported a trend towards higher diagnostic yield in non-hematologic malignancy patients (42.3%, 41/97) compared to hematologic malignancy patients (29.4%, 55/187, P=0.021) (33). Diagnostic yield was also higher among neutropenic patients compared to non-neutropenic patients (41.5% vs. 24.6%, P=0.019) (33). However, this was not a consistent finding across studies as one study reported lower yield in patients with severe neutropenia (32). One retrospective study also showed higher detection of viruses in non-neutropenic patients and both higher bacterial and viral detection in patients with hematopoietic stem cell transplants (20). Etiology of immunosuppression was otherwise not significantly associated with diagnostic yield for other studies (19,34). In addition, one study reported increased detection of Aspergillus spp. amongst neutropenic patients compared to non-neutropenic patients (12.1% vs. 4.5%, P=0.0489) (18).
Clinical/radiological presentation
Compared to asymptomatic patients, the diagnostic yield was higher in those with symptoms (61.3% vs. 29.6%, P=0.007) of fever and chest symptoms such as cough, sputum, shortness of breath and pleuritic chest pain (35). Chest computed tomography findings of consolidation, ground-glass opacities or tree-in-bud infiltrates were significantly associated with increased diagnostic yield compared to nodular and reticular infiltrates (61.2% vs. 36.5%, P=0.006) (35). Two further studies found no statistically significant difference in yield between focal or diffuse radiographic changes (10,19).
In one study on mixed etiology of immunocompromised patients, diagnostic yield was reported to be inversely proportional to duration of empiric antibiotic treatment (34). Diagnostic yield after antibiotic therapy for 3 days or less was 63.4% and yield was found to decrease as antibiotic duration increased to 14 days (57.6%) and more than 14 days (34.4%) (34). This trend of higher diagnostic yield with BAL done earlier in the course of disease was also observed in another study on bone marrow transplant patients that found significantly higher BAL yield within 4 days of presentation of pulmonary infiltrates compared to >4 days (73% vs. 31%, P<0.001) (32). BAL yield was highest within 24 hours (75%) and declined to only 14% at 10 days (32). Samples obtained from later BALs yielded a higher proportion of multidrug resistant pathogens (27% vs. 3%, P<0.001) and were more likely to be polymicrobial (30% vs. 10%, P=0.01) (32).
Complication rate of BAL
Overall complication rate of BAL was also reported in 11 studies with an average of 10.4%, ranging from 1% to 52% (Table 2). Most complications that were self-limiting such as transient hypoxemia, sinus tachycardia and limited airway bleeding (17,18,33). The rate of major adverse events including high flow oxygen requirement or mechanical ventilation, arrhythmias, hypotension needing vasopressors and severe bleeding was <5% (17). One case of death within 24 hours following bronchoscopy was reported (17). No factors that predicted either an increased or decreased adverse event rate were reported.
Discussion
The data reaffirms that despite having a common clinical presentation, immunocompromised patients are a diverse group of patients and this has made interpretation of the BAL data challenging. Only 4 of the 23 studies surveyed explicitly included HIV patients and there was a variety of etiologies of immunosuppression among patients. The differences in availability of investigations at different laboratories also impacts the ability to identify different organisms, affecting BAL diagnostic yield.
Although a wide range of diagnostic yields has been reported, it appears that on average 51.1% of immunocompromised patients with pulmonary infiltrates undergoing BAL will get a confirmed diagnosis. Post-procedural treatment modification serves as an indicator of clinical utilization of BAL findings in patient management and the average rate of this was 44.4% (Table 2). Positive BAL results with no change in management may have been the result of detection of airway commensals or organisms that were already covered by empiric antimicrobial therapy. Other identified organisms such as viruses may have no specific treatment and require only supportive therapy.
The average diagnostic yield data serves as important information that should be communicated to patients in consent taking for bronchoscopy especially in view of the average complication rate being 10.4%. The complication rate of BAL reflects the risk of flexible bronchoscopy performed under moderate sedation in this population. Data from literature reviewed in this paper has supported the use of BAL for investigating pulmonary infiltrates early in the course of disease, when clinical and radiological signs suggest an infectious etiology or when the patient has a non-hematologic malignancy. However, these findings have not been consistently replicated and it is difficult to draw definite conclusions on optimal patient selection. This is despite the fact that >3,000 bronchoscopic procedures have been reported on the subject in the published data since the year 2000.
Conclusions
This paper provides a review of recent medical literature on the use of BAL for the diagnosis of pulmonary infiltrates in immunocompromised patients and has highlighted differing findings in the available data. There is also significant variation in the etiologies of immunosuppression studied and diagnostic yields and complication rates reported encompass a broad range of values. A confirmed diagnosis may be established with BAL in approximately 50% of cases and may alter clinical management. Complications are usually self-limiting and occur in about 10% of cases. Improved understanding of the factors that influence diagnostic yield and complication rates may optimize the patient selection for this procedure to maximize benefit and minimize adverse outcomes. However, data is currently lacking and should be the focus of future research given the morbidity and mortality faced by immunocompromised patients presenting with pulmonary infiltrates.
Acknowledgements
This study was supported by the SingHealth Duke-NUS Academic Clinical Programme and the AM-ETHOS Duke-NUS Medical Student Fellowship (Duke-NUS MSF) Award 2017.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jepson SL, Pakkal M, Bajaj A, et al. Pulmonary complications in the non-HIV immunocompromised patient. Clin Radiol 2012;67:1001-10. [Crossref] [PubMed]
- Rañó A, Agustí C, Jimenez P, et al. Pulmonary infiltrates in non-HIV immunocompromised patients: a diagnostic approach using non-invasive and bronchoscopic procedures. Thorax 2001;56:379-87. [Crossref] [PubMed]
- Rañó A, Agusti C, Benito N, et al. Prognostic factors of non-HIV immunocompromised patients with pulmonary infiltrates. Chest 2002;122:253-61. [Crossref] [PubMed]
- Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax 2013;68 Suppl 1:i1-44. [Crossref] [PubMed]
- Hong KS, Choi EY, Park DA, et al. Safety and Efficacy of the Moderate Sedation During Flexible Bronchoscopic Procedure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine (Baltimore) 2015;94:e1459. [Crossref] [PubMed]
- Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 2012;185:1004-14. [Crossref] [PubMed]
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014;58:309-18. Erratum in: Clin Infect Dis 2014;59:144. [Crossref] [PubMed]
- Baughman RP. The lung in the immunocompromised patient. Infectious complications Part 1. Respiration 1999;66:95-109. [Crossref] [PubMed]
- Rosenow EC 3rd, Wilson WR, Cockerill FR 3rd. Pulmonary disease in the immunocompromised host. 1. Mayo Clin Proc 1985;60:473-87. [Crossref] [PubMed]
- Peikert T, Rana S, Edell ES. Safety, diagnostic yield, and therapeutic implications of flexible bronchoscopy in patients with febrile neutropenia and pulmonary infiltrates. Mayo Clin Proc 2005;80:1414-20. [Crossref] [PubMed]
- Joos L, Chhajed PN, Wallner J, et al. Pulmonary infections diagnosed by BAL: a 12-year experience in 1066 immunocompromised patients. Respir Med 2007;101:93-7. [Crossref] [PubMed]
- Azoulay E, Mokart D, Lambert J, et al. Diagnostic strategy for hematology and oncology patients with acute respiratory failure: randomized controlled trial. Am J Respir Crit Care Med 2010;182:1038-46. [Crossref] [PubMed]
- Rabbat A, Chaoui D, Lefebvre A, et al. Is BAL useful in patients with acute myeloid leukemia admitted in ICU for severe respiratory complications? Leukemia 2008;22:1361-7. [Crossref] [PubMed]
- Seneviratna A, O'Carroll M, Lewis CA, et al. Diagnostic yield of bronchoscopic sampling in febrile neutropenic patients with pulmonary infiltrate and haematological disorders. Intern Med J 2012;42:536-41. [Crossref] [PubMed]
- Eyüboğlu FÖ, Kupeli E, Bozbas SS, et al. Evaluation of pulmonary infections in solid organ transplant patients: 12 years of experience. Transplant Proc 2013;45:3458-61. [Crossref] [PubMed]
- Kupeli E, Akcay S, Ulubay G, et al. Diagnostic utility of flexible bronchoscopy in recipients of solid organ transplants. Transplant Proc 2011;43:543-6. [Crossref] [PubMed]
- Gilbert CR, Lerner A, Baram M, et al. Utility of flexible bronchoscopy in the evaluation of pulmonary infiltrates in the hematopoietic stem cell transplant population -- a single center fourteen year experience. Arch Bronconeumol 2013;49:189-95. [PubMed]
- Hummel M, Rudert S, Hof H, et al. Diagnostic yield of bronchoscopy with bronchoalveolar lavage in febrile patients with hematologic malignancies and pulmonary infiltrates. Ann Hematol 2008;87:291-7. [Crossref] [PubMed]
- Jain P, Sandur S, Meli Y, et al. Role of flexible bronchoscopy in immunocompromised patients with lung infiltrates. Chest 2004;125:712-22. [Crossref] [PubMed]
- Kim SW, Rhee CK, Kang HS, et al. Diagnostic value of bronchoscopy in patients with hematologic malignancy and pulmonary infiltrates. Ann Hematol 2015;94:153-9. [Crossref] [PubMed]
- Danés C, González-Martín J, Pumarola T, et al. Pulmonary infiltrates in immunosuppressed patients: analysis of a diagnostic protocol. J Clin Microbiol 2002;40:2134-40. [Crossref] [PubMed]
- Reichenberger F, Dickenmann M, Binet I, et al. Diagnostic yield of bronchoalveolar lavage following renal transplantation. Transpl Infect Dis 2001;3:2-7. [Crossref] [PubMed]
- Hohenadel IA, Kiworr M, Genitsariotis R, et al. Role of bronchoalveolar lavage in immunocompromised patients with pneumonia treated with a broad spectrum antibiotic and antifungal regimen. Thorax 2001;56:115-20. [Crossref] [PubMed]
- Taggart S, Breen R, Goldsack N, et al. The changing pattern of bronchoscopy in an HIV-infected population. Chest 2002;122:878-85. [Crossref] [PubMed]
- Bissinger AL, Einsele H, Hamprecht K, et al. Infectious pulmonary complications after stem cell transplantation or chemotherapy: diagnostic yield of bronchoalveolar lavage. Diagn Microbiol Infect Dis 2005;52:275-80. [Crossref] [PubMed]
- Hofmeister CC, Czerlanis C, Forsythe S, et al. Retrospective utility of bronchoscopy after hematopoietic stem cell transplant. Bone Marrow Transplant 2006;38:693-8. [Crossref] [PubMed]
- Vélez L, Correa LT, Maya MA, et al. Diagnostic accuracy of bronchoalveolar lavage samples in immunosuppressed patients with suspected pneumonia: analysis of a protocol. Respir Med 2007;101:2160-7. [Crossref] [PubMed]
- Boersma WG, Erjavec Z, van der Werf TS, et al. Bronchoscopic diagnosis of pulmonary infiltrates in granulocytopenic patients with hematologic malignancies: BAL versus PSB and PBAL. Respir Med 2007;101:317-25. [Crossref] [PubMed]
- Burger CD. Utility of positive bronchoalveolar lavage in predicting respiratory failure after hematopoietic stem cell transplantation: a retrospective analysis. Transplant Proc 2007;39:1623-5. [Crossref] [PubMed]
- Cordani S, Manna A, Vignali M, et al. Bronchoalveolar lavage as a diagnostic tool in patients with hematological malignancies and pneumonia. Infez Med 2008;16:209-13. [PubMed]
- Kuehnhardt D, Hannemann M, Schmidt B, et al. Therapeutic implication of BAL in patients with neutropenia. Ann Hematol 2009;88:1249-56. [Crossref] [PubMed]
- Shannon VR, Andersson BS, Lei X, et al. Utility of early versus late fiberoptic bronchoscopy in the evaluation of new pulmonary infiltrates following hematopoietic stem cell transplantation. Bone Marrow Transplant 2010;45:647-55. [Crossref] [PubMed]
- Sampsonas F, Kontoyiannis DP, Dickey BF, et al. Performance of a standardized bronchoalveolar lavage protocol in a comprehensive cancer center: a prospective 2-year study. Cancer 2011;117:3424-33. [Crossref] [PubMed]
- Kottmann RM, Kelly J, Lyda E, et al. Bronchoscopy with bronchoalveolar lavage: determinants of yield and impact on management in immunosuppressed patients. Thorax 2011;66:823. [Crossref] [PubMed]
- Brownback KR, Simpson SQ. Association of bronchoalveolar lavage yield with chest computed tomography findings and symptoms in immunocompromised patients. Ann Thorac Med 2013;8:153-9. [Crossref] [PubMed]
- Svensson T, Lundstrom KL, Hoglund M, et al. Utility of bronchoalveolar lavage in diagnosing respiratory tract infections in patients with hematological malignancies: are invasive diagnostics still needed? Ups J Med Sci 2017;122:56-60. [Crossref] [PubMed]
- Sakata KK, Klassen CL, Bollin KB, et al. Microbiologic yield of bronchoalveolar lavage specimens from stem cell transplant recipients. Transpl Infect Dis 2017.19. [PubMed]



