
Computed tomography-guided dye localization for deeply situated pulmonary nodules in thoracoscopic surgery
Introduction
Because of the increasing worldwide adoption of lung cancer screening in asymptomatic adults through the use of low-dose computed tomography (CT) with high-resolution imaging modalities, the identification of small undiagnosed pulmonary nodules is increasing (1,2). For those ground-glass opacity (GGO)-dominant lung nodules that do not disappear during follow-up, a definite pathological diagnosis is necessary. However, conventional percutaneous CT-guided biopsy has a limited diagnostic yield, especially for small lesions or those that are GGO-dominant (3). Video-assisted thoracoscopic surgical resection (VATS) contributes to both accurate early diagnosis and curative intention (1). To avoid unnecessary pulmonary resection, precise localization techniques are crucial. For some small and deep nodules, locating the nodule position accurately is challenging, especially in thoracoscopic surgery (4). The conventional tactile location method is no longer deemed reliable for such impalpable nodules due to its low detection rate, even in thoracotomy-based approaches (5). Therefore, several localization methods, most of which are image-guided, have been used to locate nodules deep in the pulmonary parenchyma. These methods can be classified into three major groups: (I) image-guided percutaneous placement of dye, hooks, coils, or radiopaque markers; (II) transbronchial dye injection or fiducial placement under bronchoscopy- or fluoroscopy-guided approaches; and (III) intraoperative ultrasound (6).
The dye localization of pulmonary nodules, using methylene blue, was first reported on 1994 (7). This procedure has several advantages. For example, the dye can be injected either through the endobronchial (8) or transthoracic route guided by fluoroscopy or by using conventional multidetector CT (9,10); this can achieve a high success rate and short procedure time (6). Moreover, this procedure is not limited by the anatomical position of nodules and radiologists and surgeons are not exposed to radiation (6). The major disadvantage of this procedure is the rapid diffusion of methylene blue dye into the surrounding lung parenchyma, resulting in a blurred injection area. Furthermore, the subsequent surgery must start immediately after lung marking (11). It has been reported that several techniques can be used that avoid dye diffusion, such as by mixing the methylene blue dye with other compounds, [e.g., atelocollagen (12) and autologous blood (13)]. Lin et al first reported the use of patient blue vital (PBV) dye instead of methylene blue in preoperative localization followed by uniportal thoracoscopic surgery, and the results revealed that this technique has both high accuracy and safety (14). Although dye localization is one advantage of PBV dye, its applicability to nodules deeper in the lung parenchyma without rapid diffusion requires further investigation. This study aimed to evaluate the role of preoperative PBV dye localization for an undiagnosed nodule deeply situated in the lung parenchyma followed by minimally invasive lung resection.
Methods
Study design and patients
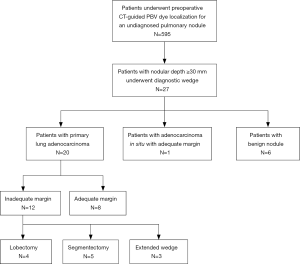
From July 2013 to December 2016, a total of 595 patients with incidentally found, indeterminate, and previously undiagnosed pulmonary nodules underwent preoperative CT-guided PBV dye localization followed by thoracoscopic resection at National Taiwan University Hospital. All patients were treated by a single surgical team composed of the same surgeons, radiologists, and anesthesiologists who had engaged in long-term collaboration using the same patient care protocols. In our institution, the surgical indications of these patients included enlargement of the nodule size on follow-up CT images or persistence of a nodule with a solid component larger than 5 mm. We retrospectively reviewed all the 595 patients’ final CT images before operation by using a commercially available viewer (Impax 5.2; Agfa HealthCare, Mortsel, Belgium). All the nodules were examined in the lung window settings (window level: −500 HU, window width: 1,500 HU) from every direction, including axial, sagittal, and coronal views). A deeply situated nodule was defined as one for which the distance between the nearest visceral pleural surface and the nodular margin was ≥30 mm. Of the 595 patients, 27 met the criteria of having deeply situated nodules and were enrolled in this study. Figure 1 showed the algorithm of patient selection and management. This study was approved by the Research Ethics Committee of National Taiwan University Hospital (project approval number: 201607088RINA).
CT-guided PBV dye localization
All the patients’ preoperative CT images were reviewed by radiologists and surgeons to consider if their nodules would be excessively difficult to intraoperatively visualize during thoracoscopy and would require preoperative localization. Each patient indicated for CT-guided dye met at least one of the following criteria: (I) pure ground-glass nodules (GGNs); (II) partial solid nodules with a diameter of >10 and ≤20 mm; (III) any nodules with a diameter of ≤10 mm; (IV) deep nodules with a distance from the nodule to the pleural surface of >20 mm (15).
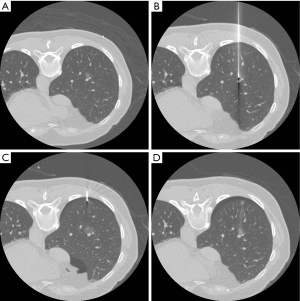
All localization procedures were performed in the CT room by an experienced radiologist using a 16-slice CT scanner (GE LightSpeed; GE Healthcare, Milwaukee, Wisconsin, USA), with a low-dose exposure and thin slice protocol (1.25 mm thickness, 1.3 pitch, 0.7 s/rotation, 120 kV, 50 mA). Details of the procedure have been described in several studies (9,10,14,15). Briefly, the patient could be in the supine, prone, or lateral decubitus position with the side with the lesion facing upwards to ensure the optimal planned needle path (Figure 2A). After it was disinfected, a 22-gauge Chiba needle was positioned toward the target lesion under CT-guidance until the tip of the needle reached the lesion. Two doses of PBV dye (2.5%; Guerbet, Aulnay-sous-Bois, France) were injected slowly; the first does was injected near the target lesion deep inside the lung parenchyma and the second dose was administered near the surface of the pleura that the needle entered (Figure 2B,C). In total, 0.2–0.3 mL of PBV dye was injected (0.1–0.15 mL for each area). One last CT image was captured before the end of the procedure to ensure that the nodule, especially the deep margin, was fully covered by the dye (Figure 2D), and to screen for potentially life-threatening pneumothorax or hemothorax. After the procedure, the patient was sent back to the general ward for surgery.
Thoracoscopic surgery
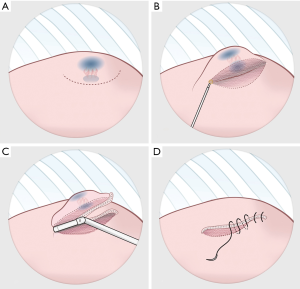
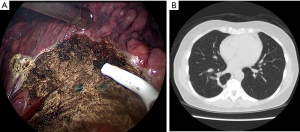
The operation could be performed through either multiportal or uniportal VATS (14,15), depending on the complexity of the procedure. Although it is easy to recognize the blue area in the pleural surface, occasionally nodules deep inside the parenchyma could not be resected using the trans-fissure approach. Such a resection is difficult because the thick lung parenchyma surrounding a deep nodule does not allow for the complete closure of staples. In such cases, the visceral pleura was opened through electrocautery along the side of the marked area in the surface (Figure 3A), and dissection through the parenchyma along the marked blue area could be performed in in the deep lung parenchyma (Figure 3B). Once the stapler could be used, diagnostic wedge resection was performed (Figure 3C). It was crucial to ensure that all of the marked blue area was resected. The rough surface of the lung parenchyma was closed by suturing it with 4-0 prolene (Figure 3D). The target lesion could be identified in the resected specimen and sent to be frozen for pathological examination. Figure 4A presents the thoracoscopic view during dissection around the parenchyma. Once the malignancy was confirmed, segmentectomy, lobectomy or the additional wedge resection could be undertaken to obtain an adequate section margin. Mediastinal lymph nodal dissection was performed in every patient with primary lung cancer. We also routinely performed intercostal nerve blocks from the third to the eighth intercostal nerves using bupivacaine (0.5%, 1.5 mL for each intercostal space) (15,16). At the end of surgery, lung inflation was performed to investigate the air leaks, and a chest tube was placed through the camera port into the posterior aspect of the thoracic cavity.
Data collection and statistical analysis
The clinical characteristics of the nodules were collected based on the final CT images before operation (usually within 2 weeks before surgery), including nodular location, maximum diameter, and nodular depth (the distance between the nearest visceral pleural surface and margin of the nodule). The density of the GGO percentage was determined using the consolidation/tumor ratio (C/T ratio; the ratio of the maximum diameter of the consolidation divided by the maximum nodular diameter). Patient demographics, clinical characteristics, hospitalizations, procedure results and surgical outcomes were collected from the patients’ medical records. Continuous variables were presented as medians and interquartile range (IQR), while categorical variables were presented as counts (percentages). All descriptive statistics were calculated using SPSS version 22.0 (IBM SPSS Statistics, Chicago, IL, USA).
Results
From July 2013 to December 2016, 27 consecutive patients (16 women, median age: 62 years, IQR, 55–67 years) with deep-situated undiagnosed pulmonary nodules underwent preoperative CT-guided PBV dye localization followed by thoracoscopic resection of the nodule. Of the patients, 25 were never-smokers (92.6%) and all (N=27) patients had normal lung function. More than half of the nodules were located in the right upper lobe (N=14, 51.9%). The median nodular maximum diameter (including the GGO part) in preoperative CT images was 11 mm (IQR, 9–13 mm) with a median depth of 31.6 mm (range, 30.0–48.6 mm; IQR, 30.0–34.0 mm). The median depth-to-size ratio (DS ratio) was 3.28 (IQR, 2.50–3.80). Of the 27 nodules, 8 were pure GGNs, 3 were pure solid nodules, and 16 were partially solid nodules; the median C/T ratio was 0.32 (IQR, 0–0.85). Table 1 presents the patient demographics and clinical characteristics.
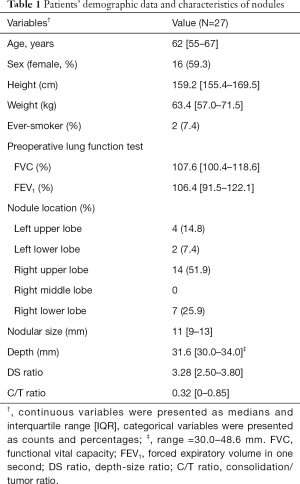
Full table
Details of the CT-guided localization and surgical results are listed in Table 2. The median distance traversed by the needle, from the pleura to the nodule, was 36.0 mm (IQR, 28.0–43.2 mm). Only asymptomatic complications were noted after localization, including mild pneumothorax (N=10, 37.0%) and trance parenchymal hemorrhage (N=17, 63.0%). All the patients tolerated the treatment well without requirement for invasive intervention after localization, and no patient had unstable hemodynamics during anesthesia and positive ventilation. The diagnostic yield of CT-guided dye localization following diagnostic wedge resection was 100%. The final pathology confirmed the diagnoses of primary adenocarcinoma of the lung (N=20), adenocarcinoma in situ (N=1), and benign nodules (N=6). The benign nodules included pulmonary tuberculosis (N=1), inflammatory diseases (N=1), nodular parenchyma amyloidosis (N=1), schwannoma (N=1), and minute pulmonary meningothelial-like nodules (N=2). Once an analysis of the frozen section confirmed a diagnosis of primary lung cancer, the section margin was examined immediately. To obtain an adequate section margin, additional lung resection was performed in 12 patients (57.1%), including completion lobectomy (N=4), segmentectomy (N=5), and extended wedge resection (N=3). The median operative time was 125 min (IQR, 95–165 min). The median duration of chest tube drainage was 2 days (IQR, 1–2 days) and the median hospital stay was 3 days (IQR, 3–4 days). Regular follow-up CT images were captured every 6 months for 21 patients with primary lung cancer, and all of the patients were cancer-free after a median follow-up of 39.0 months (IQR, 29.5–50.0 months). Figure 4B showed the follow-up CT images 2 years after operation.
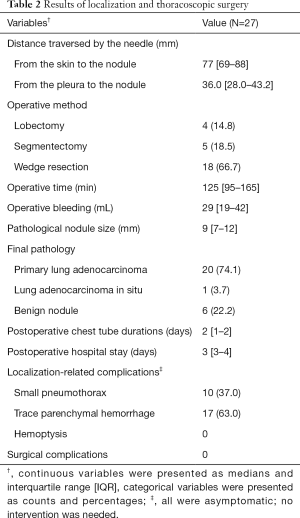
Full table
Discussion
VATS has been widely used as a preferred treatment option for resection of an incidentally found indeterminate pulmonary nodule, for both in-tissue diagnosis and curative surgery. VATS can provide a more accurate diagnosis than conventional percutaneous or bronchoscopic biopsy. However, VATS has limitations, in that its detection of nodules depends on the size and depth of the target lesion. Suzuki et al. reported a 63% of failure rate to detect nodules ≤10 and >5 mm deep in the lung parenchyma without localization in VATS (17). Therefore, a precise localization approach can help determine the exact location of the nodule so that unnecessary parenchyma loss during resection can be avoided. However, no consensus has been reached regarding universally preferred methods for localization: each method has advantages and disadvantages.
Management of a deep-situated pulmonary nodule is challenging. No common definition of deep nodules is available. Most researchers use distance between the tumor border to the nearest visceral pleura as the tumor depth, with a nodule with a depth of ≥20 mm considered as deep (18). Several studies have reported that localization of a nodule with a depth of approximately 20–25 mm could be feasible and effective (9,18-20). Nodules with a depth of >3 mm are usually considered deep and an anticipated lobectomy was required (9). In this study, we attempted to evaluate the role of localization for an extremely deep nodule to expand the use of localization to deeper nodules.
The successful localization of a deep nodule relies on both marks on superficial pleura and locations inside the parenchyma, which can provide details of the exact location and depth, respectively. Superficial marks provide surgeons with the exact location of a nodule, and deep marks informs the surgeon of the depth of the tissue that should be taken. Some metallic materials (hook-wire, microcoils, and fiducial markers), water-insoluble contrast media (lipiodol and barium), and fluorescent indocyanine green (ICG) can be used in the localization of nodules within the lung parenchyma. However, these techniques have distinct disadvantages. The major drawback of hook-wires, the most common material for localization, is that they can become dislodged from the target location. The reported dislodgement rate is approximately 2.4–6.9%, and it can occur during patient transportation, lung deflation during anesthesia, and lung manipulation during surgery (6). Seo et al. reported that the distance between the hook-wire tip and pleural surface is the most crucial factor for a successful localization (21). Additionally, hook-wire carry the potential risks of inducing hemopneumothorax and major air embolism (22). Furthermore, a hook-wire that is left protruding extracorporeally may not be accepted by some patients, owing to discomfort or fear during waiting time before surgery. Microcoil and fiducial markers are deployed into the lung parenchyma, which is not visualized directly, and fluoroscopic guidance is required at the time of resection, leading to additional radiation exposure in patients and practitioners (23-27). Additionally, a large microcoil, such as one 15–80 mm in length and 4–5 mm in diameter, could confer an increased risk of gas embolism compared with hook-wires (11). Water-insoluble contrast medium (lipiodol and barium) (11,28,29) also requires intraoperative fluoroscopic guidance and further radiation exposure. Some researchers have expressed concern that barium can affect pathological findings caused by inflammatory findings and barium itself, and air embolism can be result from intravascular injection of water-insoluble lipiodol (29). A novel technique, near-infrared imaging (NIR), has been used to detect pulmonary nodules after injection of ICG (18,30,31). In this technique, laser light and NIR emission light are both used to activate ICG within the lung parenchyma, which provides surgeons with imaging data in real time that is easy to comprehend, without exposure the radiation. However, the tissue penetration of NIR fluorescence is limited to tissues at depths of more than 24 mm, which may not be applicable in the localization of deeper nodules (30).
Localization using methylene blue dye is a straightforward procedure and has a high success rate. Kleedehn et al. reported that compared with hook-wire insertion, methylene blue injection is equally efficacious but has less complications (32). However, methylene blue has certain limitations; it can easily diffuse over the pleural surface, rendering it difficult to identify alongside extensive anthracotic pleural pigments. Moreover, methylene blue does not mark the depth of a lesion (19). Lin et al. were the first to report using of PBV dye instead of methylene blue for localization (14), with a high success rate and safety. Their results demonstrated that PBV dye caused minimal diffusion even after an average waiting time of 4 hours between localization and surgery. Furthermore, PBV dye is applicable in deeper areas of the parenchyma, meaning the lower margin of the nodule is clearly visible during operations. One of the most substantial disadvantages of PBV dye is the risk of a lethal allergic reaction. However, such a reaction is extremely rare and has never reported in a thoracic lung resection (9,10,14-16,33,34).
The major concern of sublober resection for early-stage lung cancer is the provision of a clean section margin. Our method of opening the visceral pleural facilitates applying the stapler, even in deep, thick, parenchyma tissue. Because the nodule is covered by PBV dye, we were only required to ensure that all the tissues with blue pigments were resected. This is considerably advantageous for preserving more of the lung parenchyma, especially for benign nodules. In our study, no prolonged air leaks were noted postoperatively and chest tubes were removed within 3 days. Figure 4B shows that the favorable healing of a lung after this pleural opening procedure. PBV dye does not affect tissue diagnosis in specimens, and it is not difficult to identify the nodule, even against a blue background. Our results showed that the diagnostic yield reached 100%. Furthermore, all the 21 patients were cancer-free after a median follow-up of 3 years, suggesting that this procedure could considerably be advantageous for both preserving the lung parenchyma and curative intention.
Our study has several limitations. First, this was a retrospective study conducted by a single surgical team from an individual institution with a period of more than 2 years. Patient selection and time-trend biases were inevitable. Moreover, the relatively small sample size was a concern. Nevertheless, our study demonstrated that PBV dye localization for deep nodules is a straightforward, safe, and feasible procedure. Further research is required to apply this technique in more patients, and this technique should be compared with other established localization methods to evaluate the diagnostic yield, safety, and cost effectiveness of these different methods.
Conclusions
Our study revealed that preoperative, percutaneous CT-guided PBV dye localization for an undiagnosed nodule at a depth of more than 30 mm could be a safe and feasible procedure. Combined with VATS, diagnostic wedge resection could be performed with less invasiveness. Furthermore, it was considerably advantageous for preserving the lung parenchyma, especially for benign nodules. This localization technique should be compared with other established methods to evaluate its relative diagnostic yield, safety, and cost effectiveness.
Acknowledgements
Funding: This work was supported by research grants from National Taiwan University Hospital, Taipei, Taiwan (NTUH-MS-419 to JS Chen).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Research Ethics Committee of National Taiwan University Hospital (project approval number: 201607088RINA).
References
- Krochmal R, Arias S, Yarmus L, et al. Diagnosis and management of pulmonary nodules. Expert Rev Respir Med 2014;8:677-91. [Crossref] [PubMed]
- Humphrey LL, Deffebach M, Pappas M, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive services task force recommendation. Ann Intern Med 2013;159:411-20. [Crossref] [PubMed]
- Shimizu K, Ikeda N, Tsuboi M, et al. Percutaneous CT-guided fine needle aspiration for lung cancer smaller than 2 cm and revealed by ground-glass opacity at CT. Lung Cancer 2006;51:173-9. [Crossref] [PubMed]
- Kohno T, Fujimori S, Kishi K, et al. Safe and Effective Minimally Invasive Approaches for Small Ground Glass Opacity. Ann Thorac Surg 2010;89:S2114-7. [Crossref] [PubMed]
- Migliore M, Fornito M, Palazzolo M, et al. Ground glass opacities management in the lung cancer screening era. Ann Transl Med 2018;6:90. [Crossref] [PubMed]
- Lin MW, Chen JS. Image-guided techniques for localizing pulmonary nodules in thoracoscopic surgery. J Thorac Dis 2016;8:S749-55. [Crossref] [PubMed]
- Lenglinger FX, Schwarz CD, Artmann W. Localization of pulmonary nodules before thoracoscopic surgery: value of percutaneous staining with methylene blue. AJR Am J Roentgenol 1994;163:297-300. [Crossref] [PubMed]
- Sakamoto T, Takada Y, Endoh M, et al. Bronchoscopic dye injection for localization of small pulmonary nodules in thoracoscopic surgery. Ann Thorac Surg 2001;72:296-7. [Crossref] [PubMed]
- Yang SM, Ko WC, Lin MW, et al. Image-guided thoracoscopic surgery with dye localization in a hybrid operating room. J Thorac Dis 2016;8:S681-9. [Crossref] [PubMed]
- Chen PH, Hsu HH, Yang SM, et al. Preoperative Dye Localization for Thoracoscopic Lung Surgery: Hybrid Versus Computed Tomography Room. Ann Thorac Surg 2018;106:1661-7. [Crossref] [PubMed]
- Nomori H, Horio H, Naruke T, et al. Fluoroscopy-assisted thoracoscopic resection of lung nodules marked with lipiodol. Ann Thorac Surg 2002;74:170-3. [Crossref] [PubMed]
- Nomori H, Horio H. Colored collagen is a long-lasting point marker for small pulmonary nodules in thoracoscopic operations. Ann Thorac Surg 1996;61:1070-3. [Crossref] [PubMed]
- McConnell PI, Feola GP, Meyers RL. Methylene blue-stained autologous blood for needle localization and thoracoscopic resection of deep pulmonary nodules. J Pediatr Surg 2002;37:1729-31. [Crossref] [PubMed]
- Lin MW, Tseng YH, Lee YF, et al. Computed tomography-guided patent blue vital dye localization of pulmonary nodules in uniportal thoracoscopy. J Thorac Cardiovasc Surg 2016;152:535-544.e2. [Crossref] [PubMed]
- Tsai TM, Hung WT, Lin MW, et al. Computed tomography-guided dye localization prior to uniportal thoracoscopic surgery for lung nodules: A propensity score matching analysis. J Formos Med Assoc 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Tsai TM, Lin MW, Hsu HH, et al. Nonintubated uniportal thoracoscopic wedge resection for early lung cancer. J Vis Surg 2017;3:155. [Crossref] [PubMed]
- Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: Indications for preoperative marking. Chest 1999;115:563-8. [Crossref] [PubMed]
- Kostrzewa M, Kara K, Rathmann N, et al. Computed tomography-assisted thoracoscopic surgery: A novel, innovative approach in patients with deep intrapulmonary lesions of unknown malignant status. Invest Radiol 2017;52:374-80. [Crossref] [PubMed]
- Starnes SL, Wolujewicz M, Guitron J, et al. Radiotracer localization of nonpalpable pulmonary nodules: A single-center experience. J Thorac Cardiovasc Surg 2018;156:1986-92. [Crossref] [PubMed]
- Abbas A, Kadakia S, Ambur V, et al. Intraoperative electromagnetic navigational bronchoscopic localization of small, deep, or subsolid pulmonary nodules. J Thorac Cardiovasc Surg 2017;153:1581-90. [Crossref] [PubMed]
- Seo JM, Lee HY, Kim HK, et al. Factors determining successful computed tomography-guided localization of lung nodules. J Thorac Cardiovasc Surg 2012;143:809-14. [Crossref] [PubMed]
- Horan TA, Pinheiro PM, Araújo L M, et al. Massive gas embolism during pulmonary nodule hook wire localization. Ann Thorac Surg 2002;73:1647-9. [Crossref] [PubMed]
- Miyoshi T, Kondo K, Takizawa H, et al. Fluoroscopy-assisted thoracoscopic resection of pulmonary nodules after computed tomography-guided bronchoscopic metallic coil marking. J Thorac Cardiovasc Surg 2006;131:704-10. [Crossref] [PubMed]
- Toba H, Kondo K, Miyoshi T, et al. Fluoroscopy-assisted thoracoscopic resection after computed tomography-guided bronchoscopic metallic coil marking for small peripheral pulmonary lesions. Eur J Cardiothorac Surg 2013;44:e126-32. [Crossref] [PubMed]
- Lizza N, Eucher P, Haxhe JP, et al. Thoracoscopic resection of pulmonary nodules after computed tomographic-guided coil labeling. Ann Thorac Surg 2001;71:986-8. [Crossref] [PubMed]
- Sancheti MS, Lee R, Ahmed SU, et al. Percutaneous fiducial localization for thoracoscopic wedge resection of small pulmonary nodules. Ann Thorac Surg 2014;97:1914-8; discussion 1919.
- Sharma A, McDermott S, Mathisen DJ, et al. Preoperative Localization of Lung Nodules With Fiducial Markers: Feasibility and Technical Considerations. Ann Thorac Surg 2017;103:1114-20. [Crossref] [PubMed]
- Moon SW, Wang YP, Jo KH, et al. Fluoroscopy-aided thoracoscopic resection of pulmonary nodule localized with contrast media. Ann Thorac Surg 1999;68:1815-20. [Crossref] [PubMed]
- Watanabe K, Nomori H, Ohtsuka T, et al. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg 2006;132:320-4. [Crossref] [PubMed]
- Anayama T, Qiu J, Chan H, et al. Localization of pulmonary nodules using navigation bronchoscope and a near-infrared fluorescence thoracoscope. Ann Thorac Surg 2015;99:224-30. [Crossref] [PubMed]
- Ujiie H, Kato T, Hu HP, et al. A novel minimally invasive near-infrared thoracoscopic localization technique of small pulmonary nodules: A phase I feasibility trial. J Thorac Cardiovasc Surg 2017;154:702-11. [Crossref] [PubMed]
- Kleedehn M, Kim DH, Lee FT, et al. Preoperative Pulmonary Nodule Localization: A Comparison of Methylene Blue and Hookwire Techniques. AJR Am J Roentgenol 2016;207:1334-9. [Crossref] [PubMed]
- Yang SM, Lin CK, Chen LW, et al. Combined virtual-assisted lung mapping (VAL-MAP) with CT-guided localization in thoracoscopic pulmonary segmentectomy. Asian J Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Hung WT, Hsu HH, Hung MH, et al. Nonintubated uniportal thoracoscopic surgery for resection of lung lesions. J Thorac Dis 2016;8:S242-50. [PubMed]


