
Is active surveillance an option for metachronous metastatic renal cell carcinoma?
About 30% of patients treated with partial or radical nephrectomy for localized renal cell carcinoma (RCC) develop nodal or distant metastases during the follow-up (1). According to the time of recurrence, relapses are classified into early or late if occurring before or after 5 years from surgery (2). Early recurrences seem to be associated with a worse prognosis in comparison with late ones (3,4). Indeed, risk stratification according to the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) currently represents the most relevant prognosticator for patients with metastatic renal cell carcinoma (mRCC) (5). In details, patients with mRCC can be classified into good, intermediate or poor prognosis according to (I) time from diagnosis to treatment <1 year; (II) Karnofsky performance score <80%; (III) anemia; (IV) hypercalcemia; (V) thrombophilia; and (VI) neutrophilia. The absence of all previous parameters identifies patients included in the favorable-risk group. Conversely, the presence of one or two, and ≥3 prognostic factors identifies intermediate, and poor-risk categories, respectively (5).
Surgical metastasectomy, ablative techniques and/or first-line targeted therapies are the most recommended options to treat patients with single metastasis or low burden tumor (6,7). However, in 2016 the European Society for Medical Oncology guidelines introduced the possibility to manage well-selected patients with favorable disease using an active surveillance (AS) protocol (8). Postponing an active systemic treatment may avoid, or reduce, drug-related toxicity and costs of treatment without affecting its potential efficacy. Chest, abdominal and pelvic computed tomography (CT) scan at baseline, and then every 3 months during the first year, every 4 months during the second year, and every 6 months thereafter, represent the most used follow-up schedule for mRCC patients on an AS protocol. Furthermore, an annual central nervous system imaging is recommended.
In the last years, few studies analyzed the efficacy of AS in patients with mRCC. Most of them were retrospective and included a heterogeneous, small and well-selected cohort of patients followed with different schedules and criteria triggering an active treatment. Consequently, only limited evidence does exist on optimal criteria to select candidates for an AS protocol and to switch to deferred active treatment without jeopardizing cancer control.
Currently, the best available evidence is represented by a prospective phase II trial published in 2016 by Rini et al. (9). In this study, the Authors analyzed 48 patients with treatment-naïve, asymptomatic mRCC who underwent a surveillance protocol for a median time of 14.9 months. Multivariable analysis showed that a higher number of IMDC risk factors and a high number of metastatic sites predicted a short surveillance period. Of note, 22 (46%) patients died of disease during the study period. Interestingly, when investigating the potential biological basis for the good outcome of mRCC patients, Rini et al. observed that patients on AS had significantly fewer immunosuppressive cells and a higher number of interferon-γ-producing T cells than the cohort of patients who began systemic therapy immediately. This phenotype, which would favor an anti-tumor immune response, could be postulated to contribute to the relatively indolent nature of tumor growth in patients on AS.
Recently, Bimbatti et al. published a retrospective analysis of 52 patients with mRCC managed with AS in a period of about 9 years assessing whether IMDC risk class, number of metastatic sites and tumor burden (TB) changed over time, and how these changes impacted on survival (10). Obviously, the practical objective was to identify which factor may help clinicians make decisions about the early termination of AS and the start of systemic therapy. While IMDC classes were assigned according to the Heng criteria (5) and the modified Rini et al. criteria (9), TB was defined as the sum in millimeters of the longest tumor diameter of each measurable lesion.
Twelve patients were metastatic at diagnosis and 40 showed a distant progression 36 months after nephrectomy. Interestingly, at the beginning of AS, 69% of patients belonged to the good-risk IMDC class, 25% to the intermediate-risk class and only 6% to the poor-risk class. Notably, 94% of the primary tumors were clear cell RCC. Lung (56%), nodes (23%), pancreas (19%) and adrenal glands (8%) were the most commonly affected metastatic sites. Median TB was 20 mm (interquartile range, 13–44 mm).
At a median follow-up of 38.5 months, 38/52 patients (73%) stopped AS. Among these patients, 36 (69%) started a systemic therapy and two (4%) died without treatment. The median time on AS was 18.3 months. Overall, 18 (35%) patients died during the study period, and the median overall survival was 80 months.
According to the IMDC prognostic classes, the median time on AS was 20.4 months in favorable-risk group, 17.8 months in the intermediate-risk group and 5 months in the poor-risk group. Baseline IMDC class turned out to be the only independent predictor of time on AS [hazard ratio (HR) 2.15; 95% confidence interval (CI), 1.19−3.87; P=0.011]. Conversely, an increased number of metastatic sites during AS (HR 2.86; 95% CI, 1.29−6.34; P=0.01) and an increase in TB (HR 1.16; 95% CI, 1.02−1.31; P=0.02) were negative predictors of overall survival in this patient cohort.
Looking at the post-surveillance phase, among the 36 patients who began first-line therapy, 29 (80.5%) received sunitinib or pazopanib, 3 (8.5%) sorafenib and 4 (11%) cytokines. The median progression-free and overall survival were 16.6 and 42.7 months, respectively. Change in TB (HR 1.26; 95% CI, 1.07−1.48; P=0.005) and IMDC classes at the start of systemic therapy (HR 0.07; 95% CI, 0.01−0.53; P=0.01 for good- vs. poor-risk class) were independent predictors of post-surveillance overall survival.
According to these results, the Authors concluded that AS could be considered a safe option in the management of selected patients with asymptomatic good- or intermediate-risk mRCC. Moreover, they suggested that the increase in TB during the AS period must be considered as a key factor to start first-line systemic therapy according to its strong negative correlation with post-surveillance overall survival. From a practical point-of-view, each incremental millimeter in TB seems to be associated with an increased risk of post-surveillance death by 21%.
Although the Authors must be congratulated for their original contribution supporting the use of AS as an option to manage well-selected patients with early (<5 years) relapse after nephrectomy, the study deserves some considerations.
As reported by the Authors, the retrospective study design and the wide timespan could have introduced important selection bias. Moreover, the termination of AS was not established according to the Response Evaluation Criteria In Solid Tumors rules alone, but it was also determined by physician’s choice.
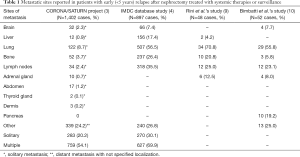
Moreover, other aspects should be considered to evaluate the exportability of this study to the current clinical practice. First, natural history and clinical behavior of synchronous metastases are quite different from those observed for metachronous metastases. Interestingly, the patients analyzed by Bimbatti et al. (10) should be considered as having an early relapse after nephrectomy, according to the most used cut-off value of 60 months (2). Patients experiencing early relapse usually exhibit different clinical and pathologic characteristic, worse response to first-line targeted therapies and worse survival than patients who develop late recurrences (4). Large multicenter studies showed that early relapses are usually characterized by multiple metastases, with single metastasis being detected only in 27% of cases (3). Sites of early relapses reported in two large retrospective studies are quite different in comparison with those reported in the Bimbatti et al.’s study (10) (Table 1). As expected in an AS series, liver metastases were absent, and the percentage of bone metastases was significantly lower in comparison with other series undergoing first-line systemic therapies. Interestingly, lung metastases were present in 56% of patients included in the University of Verona study. Patients with subcentimeter pulmonary nodules are ideal candidates for a surveillance program because they are less likely to experience disease progression (11). Patients evaluated in the University of Verona study showed a significant rate of adrenal and pancreatic metastases, which are usually more frequent in patients with late (>5 years) relapses. Both adrenal and pancreatic sites could be the expression of an indolent course of the disease with an estimated overall survival higher than 40 months (12,13).
Full table
After a median follow-up of 38.5 months, 27% of patients remained in the AS program and 73% started a first-line systemic therapy after a median time of 18 months without active treatment. Interestingly, median overall survival in the subgroup of patients undergoing deferred systemic therapies was 42.7 months, i.e., significantly higher than that reported in the COMPARZ study in the sunitinib (29.3 months) and pazopanib (28.4 months) arms (14). These data suggest that selected patients with good- and intermediate-risk class can be safely included in an AS program without compromising the efficacy of first-line targeted therapy.
Consistent results supporting AS in well-selected mRCC patients have been recently reported by Woldu et al., who analyzed retrospectively a series of 2,176 mRCC patients receiving an initial cytoreductive nephrectomy. In this study, the delayed start of targeted therapy failed to independently predict worse overall survival (15). However, in this retrospective study, delayed and late time to initiation of systemic therapy was defined as 4–6 months and >6 months, respectively.
Patients with solitary relapse after a prolonged disease-free survival after nephrectomy represent the ideal candidates for surgical metastasectomy with curative intent. Moreover, radiotherapy, ablative treatments and surgical metastasectomy could also be considered in the context of a multimodal approach in patients with favorable characteristics and low metastatic TB (16). In their original study, Bimbatti et al. included patients who received radiotherapy to targeted lesions or surgery with residual measurable disease (10). Unfortunately, the Authors did not report specific information about this interesting subgroup. According to Capitanio et al., it is possible that patients who might be candidates for AS might be those who receive the greatest extent of debulking on initial management (17).
Notably, a recent randomized, open-label, phase II trial (RESORT trial: NCT01444807) is comparing the oncologic outcomes of patients who were randomized to surveillance versus sorafenib after complete metastasectomy (18). Preliminary data presented during the 2018 American Society of Clinical Oncology Annual Meeting showed that median recurrence-free survival was 29 months in the sorafenib arm vs. 35 months in the surveillance one.
In conclusion, few studies currently support the use of AS in a well-selected subset of patients with indolent, asymptomatic, and good-risk mRCC. Although delaying systemic treatment in this selected category of patients does not seem to have negative consequences on overall survival, the dilemma persists as to whether cancer control in patients managed with an initial AS in comparison to immediate systemic therapy is compromised, and how critical the extent of initial debulking is. Indeed, metastasectomy, before or during AS, for a new small metastasis or for a low burden/low proliferating disease, should be considered within a multimodal approach in order to potentially limit the extent of progression and postpone the initiation of systemic therapy, thereby reducing toxicity and costs.
While we await further well-conducted studies to clarify the benefits and risks of AS in mRCC, the issue of whether this treatment modality is an option or an exception remains open (17).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hsieh JJ, Purdue MP, Signoretti S, et al. Renal cell carcinoma. Nat Rev Dis Primers 2017;3:17009. [Crossref] [PubMed]
- Ficarra V, Novara G. Kidney cancer: Characterizing late recurrence of renal cell carcinoma. Nat Rev Urol 2013;10:687-9. [Crossref] [PubMed]
- Brookman-May SD, May M, Shariat SF, et al. Time to recurrence is a significant predictor of cancer-specific survival after recurrence in patients with recurrent renal cell carcinoma--results from a comprehensive multi-centre database (CORONA/SATURN-Project). BJU Int 2013;112:909-16. [PubMed]
- Kroeger N, Choueiri TK, Lee JL, et al. Survival outcome and treatment response of patients with late relapse from renal cell carcinoma in the era of targeted therapy. Eur Urol 2014;65:1086-92. [Crossref] [PubMed]
- Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009;27:5794-9. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Kidney cancer. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx#site
- European Association of Urology Guidelines. Renal Cell Carcinoma. Available online: http://uroweb.org/guideline/renal-cell-carcinoma/
- Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v58-68. [Crossref] [PubMed]
- Rini BI, Dorff TB, Elson P, et al. Active surveillance in metastatic renal-cell carcinoma: a prospective, phase 2 trial. Lancet Oncol 2016;17:1317-24. [Crossref] [PubMed]
- Bimbatti D, Ciccarese C, Fantinel E, et al. Predictive role of changes in the tumor burden and International Metastatic Renal Cell Carcinoma Database Consortium class during active surveillance for metastatic renal cell carcinoma. Urol Oncol 2018;36:526.e13-8. [Crossref] [PubMed]
- Mano R, Vertosick E, Sankin AI, et al. Subcentimeter pulmonary nodules are not associated with disease progression in patients with renal cell carcinoma. J Urol 2015;193:776-82. [Crossref] [PubMed]
- Chrom P, Stec R, Bodnar L, et al. Prognostic Significance of Pancreatic Metastases from Renal Cell Carcinoma in Patients Treated with Tyrosine Kinase Inhibitors. Anticancer Res 2018;38:359-65. [PubMed]
- Takashi M, Takagi Y, Sakata T, et al. Surgical treatment of renal cell carcinoma metastases: prognostic significance. Int Urol Nephrol 1995;27:1-8. [Crossref] [PubMed]
- Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013;369:722-31. [Crossref] [PubMed]
- Woldu SL, Matulay JT, Clinton TN, et al. Incidence and Outcomes of Delayed Targeted Therapy After Cytoreductive Nephrectomy for Metastatic Renal-Cell Carcinoma: A Nationwide Cancer Registry Study. Clin Genitourin Cancer 2018;16:e1221-35. [Crossref] [PubMed]
- Dabestani S, Marconi L, Hofmann F, et al. Local treatments for metastases of renal cell carcinoma: a systematic review. Lancet Oncol 2014;15:e549-61. [Crossref] [PubMed]
- Capitanio U, Larcher A, Dell’Oglio P, et al. Re: Brian I. Rini, Tanya B. Dorff, Paul Elson, et al. Active Surveillance in Metastatic Renal-cell Carcinoma: A Prospective, Phase 2 Trial. Lancet Oncol Lancet 2016;17:1317-24: Active Surveillance in Metastatic Renal Cell Carcinoma: Option or Exception? Eur Urol 2017;71:e139-40. [Crossref] [PubMed]
- Procopio G, Cognetti F, Miceli R, et al. A randomized, open label, multicenter phase 2 study, to evaluate the efficacy of sorafenib in patients with advanced renal cell carcinoma (RCC) after a radical resection of the metastases: the RESORT trial. J Clin Oncol 2018;36;abstr 4502.


