An evaluation of adult critical result policies in haematology in a teaching hospital in China
Introduction
Critical results (CR) are test results that may signify pathophysiological states that are potentially life threatening or that could lead to irreversible damage to the patient and therefore require urgent medical attention and action (1,2). The CR concept was first proposed by Lundberg in 1972. Since then, CRs have been included as part of the clauses in CLIA’88 and have become inspection requirements by various accreditation/certification agencies such as JCI, CAP, and ISO 15189 (3-5). In China, CR reporting has been listed as one of the patient safety goals by the Chinese Hospital Association since 2007; the implementation of CR reporting is required to be monitored on a regular basis (6). The 6th edition of the JCI also requires that compliance with CR reporting should be monitored as stated in the International Patient Safety Goals (IPSG2.1) (3).
Currently, some regional investigations of CR polices and several standards, guidelines and expert consensuses on CR exist (1,7-17). These promote the standardization and consensuses of CR policies (18,19). Moreover, yearly review of CR policies, including CR reporting efficacy, indicators of quality and compliance with regulatory requirements, has been recommended (3,4,6,15). As one of the core medical policies, CR vary in different hospitals in China and those in other countries (8). Hospitals should individualize their best practices for CR policies; hence, their evaluation should be performed. However, few reports of the evaluation of CR policies were found. The evaluation of CR policies includes the following components: first, the literature is searched to provide evidence of the best practices of CR polices; second, verification and evaluation of the rationality of CR is conducted with hospital data; finally, CR compliance is investigated in terms of employee awareness and implementation of CR policies.
Haematology tests are among the most common laboratory tests, and the white blood cell (WBC) count, platelet (PLT) count and haemoglobin (HgB) level have been included as CR items. In clinical practice, CR can inform doctors that a patient may have an acute bleeding event, a new leukaemia diagnosis or relapse, a need for urgent transfusion, or risk of bleeding. The First Affiliated Hospital, College of Medicine, Zhejiang University (FHZU) has a bone marrow transplantation/leukaemia centre with a patient population with a high incidence of liver disease/cirrhosis/gastrointestinal bleeding. Thus, evaluation of CR policies in haematology is important for FHZU.
The objectives of the present study were to evaluate the consistency between adult CR policies in haematology of the FHZU and current standards, consensuses and literature; to review and analyse the distribution of CR data in haematology in 2017; to observe the CR implementation on-site; and to provide a basis for continuous improvement in the quality of CR policies for FHZU, an evaluation method of CR policies for other hospitals or other test items such as biochemistry or coagulation, and a review of big data for CR.
Methods
Study setting
The FHZU is a large-scale teaching hospital in China, with 4.21 million outpatient and emergency visits and 169 thousand discharges in 2017. It is a tertiary comprehensive adult hospital integrating medical, teaching, and research services with more than 3,200 beds. The hospital passed the JCI accreditation in 2013, and its laboratory department was accredited by ISO 15189 in 2011 and by CAP in 2015.
Haematological tests are performed in the emergency, outpatient, and inpatient departments, which are equipped with 5 XE-2100 haematology analysers, 2 XT-1800i haematology analysers and 2 SP-1000i automated slide stainers. For each day shift and night shift, there are 9 and 2 staff members on duty, respectively.
Auto-verification is used to verify the blood test results, which can provide alerts for the CR items and real-time results. Blood specimens with CRs or those requiring review do not pass auto-verification, and the reasons are indicated in the test results. Manual verification is performed by the staff; this process includes checking the instruments and quality control status, observing the quality of specimens, re-testing, and blood smearing. If blast cells/leukaemia is detected, a new morphological test item is added based on the differential of leukocytes with comments if necessary.
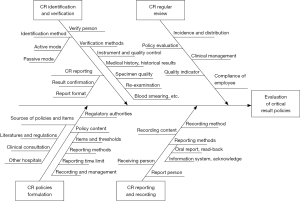
The CR items and thresholds at the FHZU are established based on the literature, consensus data, and consultation with clinical experts and are then approved by the Hospital Quality and Safety Committee. The CR items include the upper and lower alert thresholds for WBC (50.0×109/L and 1.5×109/L, respectively), upper and lower alert thresholds for PLT (1,000×109/L and 20×109/L for non-haematology departments and 10×109/L for haematology departments, respectively) and a lower alert threshold for HgB (5.5 g/dL). Some circumstances, such as alert results or large delta checks are not included in the CR report but are communicated to the clinicians by the laboratory staff. The process for CR reporting includes identification, verification, reporting, recording, and evaluation (see Figure S1). Notably, the instruments and reagents are not considered in the present study.
Literature review of policies
Relevant CR studies were searched, and the inclusion criteria were: (I) published in the past 20 years, related to CR policies, and an alert threshold was set; (II) included haematology items in the alert lists; and (III) included the CR requirements of accreditation agencies such as JCI, CAP and ISO 15189. If more than one paper was found on a similar topic, the study specified for haematological testing or the latest published study from the same region or country was chosen. The consistency between the CR policies of the FHZU and the eligible studies was evaluated.
Baseline data for the FHZU
Haematological test data for the FHZU in 2017 were retrieved from the laboratory information system as raw data, including the medical record No., department, specimen identification, test items, and results. The incidence rates of different thresholds for WBC, PLT and HgB were calculated and graphically represented. The CR distribution in different departments was determined. Thus, the rationality of the alert thresholds for haematological parameters in the FHZU was evaluated.
Policy implementation
A draft evaluation tool for on-site observation was self-designed based on the existing guidelines and consensuses on CR policies (1,3,4,15,16) and was then revised according to the opinions offered by five laboratory experts. Twenty on-duty staff members from the outpatient, emergency (night shift) and inpatient departments were investigated regarding their awareness of CR policies in haematology. Fifty CR cases each were tracked in the outpatient, emergency, and inpatient departments to cover as many staff members as possible to observe their compliance to CR policies. The data recorded during the on-site observation included the procedures for identifying, verifying, reporting, recording and clinically managing CRs. Using these data, the turn-around time (TAT) for each phase in CR implementation was calculated.
Statistical analysis
The incidences of different CR thresholds for haematological parameters, staff members’ awareness rates of CR and the compliance rates of CR polices in the FHZU with current guidelines were calculated and presented graphically using SPSS 19.0 and Excel 2013. Categorical data are represented as percentages, and TATs are represented by the median and 90th percentile (20).
Results
Evaluation of the consistency of CR polices in the FHZU with standards, consensuses and the literature
Standards, consensuses and studies on CR policies were included in the present study. After a rigorous comparison, the terms in the CR policies in the FHZU were found to be highly consistent with the consensuses and guidelines (Table 1). Notably, the International Council for Standardization in Hematology (ICSH) and the consensus from Australia were the most detailed and complete, while the requirements of CR policies by the accreditation agencies were relatively general (see Table S1 for details).
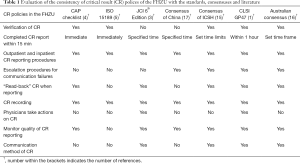
Full table
Full table
Evaluation of the consistency of alert thresholds in haematology at the FHZU with the literature
Seven studies on alert thresholds in haematology were included in the present study. The results of the comparison between the alert thresholds of the FHZU and those in the literature are listed in Table 2. As shown in the table, the lower alert thresholds of CR items in haematology were lower than the median of those in the literature, while the upper alert thresholds were higher than the median of those in the literature.

Full table
The distribution of the CRs in haematology in the FHZU
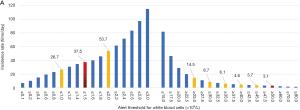
Most of the CR cases in the FHZU in 2017 were concentrated in the lower-threshold zones. As shown in Figure 1, the incidences of CR for WBC, HgB and PLT were 37.5, 18.0, and 36.9 times/day, respectively. Among the CR items in the haematology department, WBC accounted for 49.2%, and PLT accounted for 29.2% of the cases. Moreover, as indicated in Figure 1C, when the lower thresholds of PLT were set at 10.0, 20.0 and 30.0×109/L, the corresponding incidences were 16.6, 36.9 and 58.8 times/day, respectively.
Distribution of departments and number of CRs at the FHZU
In 2017, a total of 26,592 CR cases occurred, accounting for 3.8% of all laboratory specimens. The top 5 departments with the highest incidence of CRs in haematology (the number of CR cases/the total number of specimens) were the haematology department (28.7%), followed by the ICU (5.8%), emergency department (3.9%), infectious disease department (2.7%) and the surgical department (1.6%). As shown in Table 3, there were multiple CRs from the same specimen in the haematology department. Within the haematology department, 27.8% of the CRs were from the bone marrow transplantation ward. Many patients in the haematology department had CRs repeatedly. Specifically, 2,029 patients (61.9%) had CRs 1–3 times, 557 patients (17.0%) had CRs 4–10 times, and 693 patients (21.1%) had CRs 11 or more times.
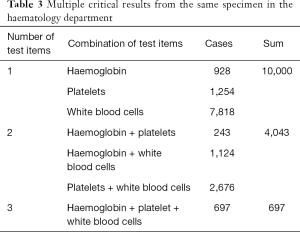
Full table
On-site evaluation of the compliance of CR policies with the guidelines
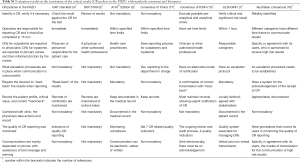
A total of 20 participants were investigated regarding their awareness of CR policies. Among them, 8 (40%) were laboratorians, 9 (45%) were supervising laboratorians and 3 (15%) were associate chief laboratorians; 5 (25%) had worked for 1–3 years, 9 (45%) had worked for 4–9 years, and 6 (30%) had worked for at least 10 years. Each participant was invited to answer questions on 9 aspects of the CR policies of the FHZU as listed in Table 1. The correct answer rate was 100%. In addition, the on-site observations of CR policy implementation by the staff in 150 CR cases were recorded as shown in Table 4.
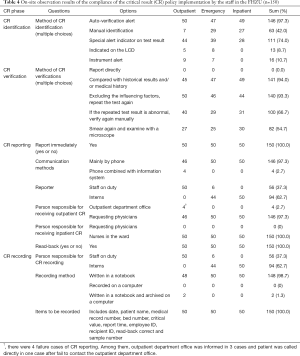
Full table
Distribution of the TATs of CRs at the FHZU
In March 2017, fifty CR cases were extracted from the outpatient, emergency, and inpatient departments respectively. The medians and 90th percentiles of the TATs for CR measurements (time of acquisition of results – specimen collection time) in the outpatient and emergency departments were 40 min/1 h 36 min and 9 min/43 min, respectively. The median and 90th percentile of the TAT for CR measurement (time of acquisition of results – specimen receipt time, excluding transportation time) in the inpatient department were 1 h 17 min/2 h 18 min. The medians and 90th percentiles of the TAT for CR identification (CR discovery time – time of acquisition of results) in the outpatient, emergency and inpatient departments were 7 min/22 min, 5 min/13 min and 16 min/54 min, respectively. The medians and 90th percentiles of the TAT for CR verification (CR reporting time – verification time) in the outpatient, emergency and inpatient departments were 1 min/4 min, 1 min/3 min and 2 min/10 min, respectively. The medians and 90th percentiles of the TATs for CR treatment by physicians (CR action time – CR reporting time) in the outpatient, emergency and inpatient departments were 38 min/23 h 1 min (5 missing cases), 1 h 48 min/13 h 24 min and 2 h 10 min/23 h 16 min (2 missing cases), respectively.
Discussion
As a core policy in the laboratory, CR policies should be reviewed and revised on a regular basis to reflect the latest requirements (1,3,17,22). When developing CR policy, a basic framework might be provided by accreditation, certification or an accrediting body, and then professional, detailed operating instructions can be specified by referring to related literature. The core requirements for the CR policies were consistent with different consensuses and literature, including the aspects of CR items and alert thresholds, CR reporting and recording procedures, and regular assessment and revision. Detailed content of the CR policy should be formulated after considering the conditions of the hospitals and laboratories, including the staff, instruments, number of specimens and special characteristics. As indicated in Table 1, the CR policies of the FHZU are consistent with the accreditation requirements, standards, consensuses or literature, thereby ensuring patient safety and effective management. Literature on CR has been increasing each year, and it is recommended to review CR policies every two years.
Currently, there are many regional or national CR surveys and consensuses, and the CR threshold might be defined or evaluated based on these data. As indicated in Table 2, the lower thresholds of CRs at the FHZU were relatively lower than, while the upper thresholds were relatively higher than those in the literature, which might be related to the fact that the FHZU is a regional medical centre in China, and the haematology department is a national key specialty department with many complicated cases. The workload of CR practice was acceptable, as indicated by regular laboratory staff surveys and clinical feedback. The incidence of CRs for haematology was highest (up to 29.7%) in the haematology department, which might be related to the fact that hospitalization of patients in these wards was strictly based on disease classification. Among the wards, the bone marrow transplantation ward was one of the branches for which blood test results were monitored on a regular basis, and thus, the incidence of repeated CRs was very high. Although no uniform alert list or threshold range was provided, the laboratory might establish individualized alert thresholds for specific patient subpopulations in consultation with the clinicians (23). Some studies have suggested that only the first CR or repeated CR satisfying the delta check should be reported. Based on guidelines such as the ICSH, some recommendations were made in the present study. First, a special CR alert range might be set for the haematology department. The WBC threshold should be decreased from 1.5×109/L to 1.0×109/L. Second, the parameters of absolute neutrophil count, acute leukaemia (>20% blast cells), acute promyelocytic leukaemia, and malarial parasites might be added to the CR items. Third, it is suggested to revise repeated CR reporting policies; only the first CR and the CR with an interval of one week should be reported. These recommendations are intended to be included in the laboratory's quality improvement programmes. After consulting with the haematology department and the department in charge, these recommendations will be reported to the Hospital Medical Quality and Safety Committee for further discussion. In addition to regular evaluation of the CR incidence, it is recommended that the CR thresholds should be strictly set in high-level hospitals, while less strict levels can be set in general medical institutions.
The on-site evaluation included the investigation of staff awareness and the on-site observation records of CR implementation. The results could be utilized as a basis for improving CR policies. The staff members engaged in haematology tests were quite familiar with the CR policies, with a correct answer rate of 100%. This is the premise of successful CR implementation. The on-site evaluation indicated that the person responsible for CR reporting varied in different departments. CR reporting and recording were mainly assigned to the interns in the laboratory of the inpatient and emergency departments, while such work was assigned to full-time staff in the laboratory of the outpatient department. The new interns might encounter many problems when managing CR reporting and recording, i.e., spending too much time searching for the contact telephone, incomplete communication, incomplete recording or recording errors. Therefore, intervention and training programmes should be provided for the interns before engaging in CR reporting (4,16). Both the guidelines and literature recommend that the CR value should be re-verified (4,15,16). As indicated in Table 4, none of the 150 CR cases observed on-site were reported directly. For most specimens, to ensure the quality of the CR verification, the CR should be compared with the historical results (94.0%) to exclude influencing factors (93.3%), perform another examination (66.7%), and review slides if necessary (54.7%). Hospitals should regularly train and evaluate the abilities of their employees to report CRs so that all personnel involved comply with the policies.
The TATs function as a quality indicator for CR reporting (4,8). The TATs of CR measurement, identification, verification, and reporting in the outpatient and emergency departments were shorter than those in the inpatient department, which might be related to the work pattern. In the outpatient and emergency departments, testing procedures were completed as soon as possible, and the TATs had a high time requirement. In the inpatient department, the TATs from specimen collection to delivery were quite long, and the specimens must be pre-treated before being measured in batches. Consequently, the TATs for CR measurement and identification were long in the wards. In addition, the TATs for clinical treatment in all three departments were quite long, requiring 0.5–1 day, which might be related to the following reasons: first, some patients require infusion of blood products, but the procedures comprising the application, preparation and infusion are very time-consuming. Second, preventive medications were administered to the patients before obtaining the results, and the clinical treatment could be postponed to some extent, or some patients may not require immediate treatment. Third, hospitalized patients could not be treated until physicians or nurses made the ward rounds on the second day.
Our study had a few limitations. First, the FHZU is a regional teaching medical centre and may not represent average hospitals in China. Second, our study was a single-centre study, and a comparison of data from other centres was lacking. Third, the sample size for on-site observations of CR implementation was relatively small, and the evaluation standards were self-designed and require further improvement.
In conclusion, the provisions of CR policies in the FHZU were highly consistent with the standards, literature, and consensuses. CR implementation by the staff also met the requirements of the CR policies. Some recommendations were made to the alert threshold for WBCs in the haematology department, the CR item list and repeated CR reporting to improve the CR reporting efficiency and ensure patient safety.
Acknowledgements
Funding: This work was supported by Zhejiang Medical and Health Science and Technology Program (2013KYA064) and Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Clinical and Laboratory Standards Institute. GP47: Management of Critical- and Significant-Risk Results, 1st Edition. Available online: http://www.clsi.org. Accessed March 5, 2017.
- Campbell CA, Horvath AR. Harmonization of critical result management in laboratory medicine. Clin Chim Acta 2014;432:135-47. [Crossref] [PubMed]
- Joint Commission International. JCI Accreditation Standards for Hospitals, 6th Edition. Available online: https://www.jointcommissioninternational.org. Accessed July 1, 2017.
- College of American Pathologists. Laboratory Accreditation Checklist. Available online: http:// www.cap.org. Accessed August 30, 2017.
- International Organization for Standardization. ISO 15189:2012. Medical laboratories– requirements for quality and competence. Geneva, Switzerland: International Organization for Standardization; 2012.
- Chinese Hospital Association. Patients safety goals for 2017 in China. Available online: http://www.cha.org.cn. Accessed January 5, 2017.
- Schapkaitz E, Mafika Z. Critical value reporting: a survey of 36 clinical laboratories in South Africa. S Afr Med J 2013;104:65-67. [Crossref] [PubMed]
- Ye YY, Zhao H, Fei Y, et al. Critical values in hematology of 862 institutions in China. Int J Lab Hematol 2017;39:513-20. [Crossref] [PubMed]
- Tillman J, Barth JH. A survey of laboratory ‘critical (alert) limits’ in the UK. Ann Clin Biochem 2003;40:181-4. [Crossref] [PubMed]
- Wagar EA, Friedberg RC, Souers R, et al. Critical values comparison: a College of American Pathologists Q-Probes Survey of 163 clinical laboratories. Arch Pathol Lab Med 2007;131:1769-75. [PubMed]
- Kopcinovic LM, Trifunović J, Pavosevic T, et al. Croatian survey on critical results reporting. Biochem Med (Zagreb) 2015;25:193-202. [Crossref] [PubMed]
- Pai M, Moffat KA, Plumhoff E, et al. Critical values in the coagulation laboratory: results of a survey of the North American Specialized Coagulation Laboratory Association. Am J Clin Pathol 2011;136:836-41. [Crossref] [PubMed]
- McFarlane A, Aslan B, Raby A, et al. Critical values in hematology. Int J Lab Hematol 2015;37:36-43. [Crossref] [PubMed]
- Howanitz PJ, Steindel SJ, Heard NV. Laboratory critical values policies and procedures: a College of American Pathologists Q-Probes Study in 623 institutions. Arch Pathol Lab Med 2002;126:663-9. [PubMed]
- Keng TB, De La Salle B, Bourner G, et al. Standardization of haematology critical results management in adults: an International Council for Standardization in Haematology, ICSH, survey and recommendations. Int J Lab Hematol 2016;38:457-71. [Crossref] [PubMed]
- Campbell C, Caldwell G, Coates P, et al. Consensus statement for the management and communication of high risk laboratory results. Clin Biochem Rev 2015;36:97-105. [PubMed]
- Clinical Laboratory Management Committee, Beijing Hospital Association. Expert Consensus of Beijing and Hebei province on standardized management of clinical laboratory critical result. Chinese Journal of Laboratory Medicine 2016;39:158-64.
- Kost GJ, Hale KN. Global trends in critical values practices and their harmonization. Clin Chem Lab Med 2011;49:167-76. [Crossref] [PubMed]
- Campbell C, Horvath A. Towards harmonisation of critical laboratory result management - review of the literature and survey of australasian practices. Clin Biochem Rev 2012;33:149-60. [PubMed]
- Pati HP, Singh G. Turnaround time (TAT): Difference in concept for laboratory and clinician. Indian J Hematol Blood Transfus 2014;30:81-4. [Crossref] [PubMed]
- Piva E, Sciacovelli L, Laposata M, et al. Assessment of critical values policies in Italian institutions: comparison with the US situation. Clin Chem Lab Med 2010;48:461-8. [Crossref] [PubMed]
- Lam Q, Ajzner E, Campbell CA, et al. Critical Risk Results - An Update on International Initiatives. EJIFCC 2016;27:66-76. [PubMed]
- Campbell CA, Horvath AR. An evidence- and risk-based approach to a harmonized laboratory alert list in Australia and New Zealand. Clin Chem Lab Med 2018;57:89-94. [PubMed]