
Can presepsin be used for screening invasive fungal infections?
Introduction
Invasive fungal infections shall be considered a major healthcare issue. Recent statistics attest that fungi are responsible for approximately 20% of all sepsis cases. Candida species (especially Candida Albicans and Candida Glabrata) are the most common fungal pathogens (~90%), whilst Aspergillus and Cryptococcus species are also frequent causes of sepsis in immunocompromised patients (1). Like all other forms of sepsis, early and accurate diagnosis is hence pivotal for establishing a timely and appropriate treatment and reversing an otherwise unfavourable outcome (2), since mortality can be as high as 80% when the appropriate treatment is delayed more than 48 hours from the onset of infection (3).
Current diagnostics of invasive fungal infections
The gold standard for diagnosing invasive fungal infection is currently represented by conventional mycological testing, thus including microscopic examination and/or blood culture (2). The first approach has some well-known drawbacks. The accuracy of microscopic diagnosis of fungal infections varies largely depending on technical skills, necessitates the support of skilled laboratory professionals for smear examination, does not allow to always accurately recognizing the fungal strains, is influenced by source or quality of samples and nature of responsible pathogens, and is plagued by an obviously low throughput (4). Blood culture has also important limitations, such as long turnaround time due to slow growth of fungi in culture systems (i.e., 1–4 days), late positivity, suboptimal diagnostic accuracy (often <50%), significant risk of false negative results when specimens are collected from patients undergoing treatment with antifungal agents, risk of environmental contamination, relatively low throughput along with biosafety hazards due to culture of mycelial forms (4). Despite the introduction of matrix-assisted laser desorption/ionisation time of flight mass spectrometry (MALDI TOF-MS) has enabled major diagnostic advancements for rapid identification of bacteria and yeasts from culture plates, its use in positive blood cultures seems still in infancy (5). The major drawbacks are represented by potential spectral interference from hemoglobin and proteins, which generates identification problems and ultimately reduces diagnostic sensitivity, as well as by the limited efficiency of current databases and software programs for complete identification of non-bacterial pathogens such as fungi (6).
Serologic testing has emerged as a valuable perspective for diagnosing patients with invasive fungal infections, since it may offset some limitations of conventional mycological testing. Nevertheless, even this approach has caveats, such as suboptimal diagnostic accuracy especially in immunocompromised patients and in those infected by rare fungal species (e.g., fusariosis, mucormycosis and scedosporiosis), relatively long turnaround time, modest throughput, possible generation of false positive results due to physiological colonization or opportunistic infections, need of clinical validation of some antigen surrogates of infection, whilst results are also strictly dependent on antibody type and kinetics (4) (Table 1).

Full table
The assessment of serum 1,3-beta-D-glucan, which has recently been proposed as useful screening test for diagnosing some invasive fungal infections, has also some diagnostic drawbacks, which are especially represented by the risk of false-positive reactions (especially in patients with bacterial infection or those undergoing dialysis) and undecided cut-offs (4). A recent meta-analysis of prospective studies also concluded that although the overall diagnostic efficiency of this test was 89% [area under the curve (AUC), 0.89; 95% confidence interval (CI), 0.86–0.91], slightly higher for candidiasis (AUC, 0.88; 95% CI, 0.83–0.98) than for aspergillosis (AUC, 0.85; 95% CI, 0.70–0.95) (7), the cumulative diagnostic sensitivity was found to be suboptimal (i.e., 0.75; 95% CI, 0.63–0.84), thus leaving up to one-fourth of patients with a risk of remaining non-diagnosed. Similar conclusions were recently published by the Third European Conference on Infections in Leukemia on the use of mannan antigen and anti-mannan antibodies for diagnosing invasive candidiasis (8), wherein the diagnostic sensitivity of these two biomarkers used alone was only 0.58 (95% CI, 0.53–0.62) and 0.59 (95% CI, 0.54–0.65), respectively, whilst their combined diagnostic sensitivity was 0.83 (95% CI, 0.79–0.87). Taken together, these findings would suggest that negative results of 1,3-beta-D-glucan, mannan antigen and anti-mannan antibodies should not be used to safely rule out invasive fungal diseases, whilst relatively long turnaround time, low throughput and limited availability of these tests in clinical laboratories are additional well-known drawbacks (Table 1). Overlapping conclusions can be drawn for other immunological assays such as glucuronoxylomannan and galactomannan (4).
Owing to the inherent drawbacks of conventional mycological testing (Table 1), the development of alternative approaches would hence be highly advisable. The development of rapid and high-throughput molecular biology techniques, especially real-time polymerase chain reaction (RT-PCR) and Multiplex, shall be seen as one of the most important breakthroughs occurred over the past decades. The molecular identification of fungal pathogen does not require live cells for success and may also enable to specifically design primers for achieving a final etiological diagnosis (4). On the other hand, some molecular biology techniques are still relatively time-consuming (i.e., >6 hours), are not always available in clinical laboratories performing urgent testing, are highly sensitive to the lysis technique used for extracting nucleic acids and to environmental contamination, can generate false positive results due to deep-seated infections, whilst the selection of optimal sample matrix (i.e., whole blood, serum or plasma) along with the limitation and poor standardization of test panels can ultimately influence their diagnostic performance (9) (Table 1).
The future of fungal sepsis biomarkers
An ideal sepsis biomarker shall allow making early diagnoses with high sensitivity and specificity, precisely identifying the pathogen, providing information on clinical course and prognosis, as well as guiding antimicrobial stewardship (10).
Procalcitonin (PCT)
Although the clinical significance of measuring PCT, the 116-amino-acid precursor of the active hormone calcitonin, is now virtually undisputed for diagnosing and managing bacterial sepsis (11), controversial evidence has been provided on its diagnostic value in patients with invasive fungal infections. More specifically, the low diagnostic sensitivity and specificity of this biomarker seemingly represent the largest barriers for its routine use in this clinical setting (12,13). Important supporting evidence on the questionable value of PCT in fungal sepsis emerged from the publication of some recent studies. Nath et al measured PCT in 300 pediatric oncology patients with suspected bloodstream infections (14), and found that the values were considerably higher in those with Gram-negative infection (n=28; 19.1 ng/mL) than in those with Gram-positive (n=11; 0.54 ng/mL) or Candida (n=4; 0.32 ng/mL) infections. Pieralli et al. measured PCT in 64 patients with Candida sepsis and in 128 with bacterial sepsis (15), reporting that values were significantly higher in those with bacterial sepsis [median, 4.48; interquartile range (IQR), 1.10–18.26 ng/mL] than in those with Candida sepsis (median, 0.73; IQR, 0.26–1.85 ng/mL; P<0.001). Notably, the AUC of PCT for distinguishing Gram-negative bacteria from fungal infections was as high as 0.94 (95% CI, 0.92–0.97). Finally, Thomas-Rüddel et al. studied 4,858 sepsis patients treated in intensive care unit (16). Overall, PCT concentration was found to be considerably higher in patients with Gram-negative bacterial sepsis (n=720; median, 26 ng/mL; IQR, 7.7–63.1 ng/mL) than in those with Gram-positive bacterial sepsis (n=855; median, 7.1 ng/mL; IQR, 2.0–23.3 ng/mL) or Candida sepsis (n=68; median, 4.7 ng/mL; IQR, 1.9–13.7 ng/mL) (both P<0.001). PCT displayed a good diagnostic efficiency for distinguishing patients with Gram-negative bacterial sepsis from those with Gram-positive bacterial sepsis or Candida sepsis (AUC, 0.69; 95% CI, 0.67–0.72). According to the cumulative findings of these three recent studies, the standardized mean difference of PCT values between patients with Gram-negative bacterial sepsis (n=876) and fungal sepsis (n=136) was 19.9 ng/mL (95% CI, 18.6–21.2; P<0.001), which means that PCT values were nearly 90% lower in the former category of patients compared to those with invasive fungal infection.
Presepsin
Presepsin, which is also known as soluble CD14 subtype (sCD14-ST), is a glycoprotein fragment mostly produced by macrophages or monocytes in response to infections (17). Presepsin can now be measured with immunochemistry techniques, which allow obtaining the first results in nearly 20–30 min after sample collection (the measuring time is ~15 min), especially when using point-of-care (POC) devices. The kinetics of presepsin is extraordinarily rapid in blood; the concentration increases within 2 hours after the onset of bacterial or fungal infections, the peak concentration is then reached between 2–4 hours, and the half-life is typically comprised between 4–6 hours (17).
The potential usefulness of measuring presepsin in sepsis diagnostics has only recently emerged (i.e., the first study appeared ~15 years ago) (18), whilst the evidence that serum PCT concentrations are increased in patients with infections and sepsis was published in The Lancet by Assicot et al., in 1993 (19), anticipated by earlier studies demonstrating that elevated values of calcitonin-related peptides are commonplace during severe infections (20,21). This relative delay is then reflected by the considerable discrepancy in the overall number of studies which have so far assessed “procalcitonin” AND “sepsis” (source PubMed, n=2,021) or “presepsin” AND “sepsis” (source PubMed, n=140). Although it is hence conceivable that the predictably larger diffusion of presepsin immunoassays would assist filling this gap, two important studies have recently contributed to provide interesting insights on the use of this biomarker for diagnosing invasive fungal infections.
Bamba et al. studied 11 consecutive patients with fungal sepsis, in whom they measured the concentration of both PCT and presepsin (22). Interestingly, although PCT values were only found to be elevated (i.e., >0.5 ng/mL) in 5/11 patients (diagnostic accuracy, 0.45; 95% CI, 0.17–0.77), two of whom with concomitant bacterial infection, presepsin level was found to be higher than the diagnostic cut-off (i.e., >500 pg/mL) in 10/11 patients (diagnostic accuracy, 0.90; 95% CI, 0.59–1.00). In the six patients with sepsis, PCT and presepsin values were found to be increased over the diagnostic threshold in 4/6 (diagnostic accuracy, 0.67; 95% CI, 0.22–0.96) and 6/6 (diagnostic accuracy, 1.00) patients, respectively. The concentration of both biomarkers was found to be correlated with the sequential organ failure assessment (SOFA) score, though the correlation was higher for presepsin (r=0.89; P<0.001) than for PCT (r=0.64; P=0.034).
Another interesting experimental study was published by Bazhenov et al. (23), who studied presepsin expression in peripheral blood mononuclear cells of 19 healthy volunteers, which were challenged with Candida albicans lysate, lipopolysaccharide or autologous serum. Notably, 24 hours after the in vitro challenge, the concentration of presepsin in the supernatant was 59.8 pg/mL (range, 29.7–140 pg/mL) in cells treated with lipopolysaccharide, 84 pg/mL (range, 38.8–133 pg/mL) in those treated with Candida albicans lysate and 34.6 pg/mL (range, 18.5–81.8 pg/mL) in those challenged with autologous serum, respectively. A similar trend was noted after 48 hours, with presepsin concentration in the supernatant of cells challenged with Candida albicans lysate remaining 1.3-fold and 2.8-fold higher than in the supernatant of cells challenged with lipopolysaccharide or autologous serum, respectively. Overall, the concentration of presepsin in the supernatant was significantly higher after challenge with Candida albicans lysate than with lipopolysaccharide (P<0.05) or autologous serum (P<0.001).
Conclusions
According to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3), sepsis is currently defined as a “life-threatening organ dysfunction caused by dysregulated host response to infection” (24). This updated definition underscores the clinical importance of the “non-homeostatic” host response to an infection rather than the infection per se, so that the concepts of “bacteraemia”, “fungemia” and even of “bloodstream infection” shall be no longer used as synonyms for sepsis. The first obvious consequence is that the measurement of biomarkers which essentially reflect a disproportionate “non-homeostatic” host response would appear more efficient for initial screening of patients with sepsis compared to the direct isolation or identification of microorganisms from blood. In fact, a systemic biological response, up to systemic inflammatory response syndrome (SIRS) and multi organ failure, can also occur in patients with severe but localized infections, even before microorganisms have massively entered the bloodstream (Figure 1).
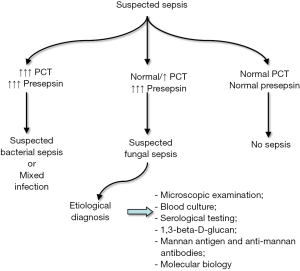
The preliminary albeit intriguing findings of the two studies investigating the role of presepsin in invasive fungal infections (22,23), along with some notable technical advantages (Table 1), conceivably pave the way to developing algorithms based on results of both PCT and presepsin testing (measured in combination for screening and differential diagnosis of patients with sepsis), which should then be validated in real life scenarios. Concomitantly increased values of PCT and presepsin would thus appear suggestive of bacterial sepsis (especially Gram-negative bacterial sepsis) or mixed infection, non-diagnostic values of both biomarkers may enable to safely rule out sepsis of both bacterial or fungal origin, whilst a disproportionate increase of presepsin values combined with normal or only modestly elevated PCT concentration would be suggestive for invasive fungal infection. Additional tests, such as those reported in Table 1, can then be planned for achieving a more accurate etiological diagnosis (Figure 1).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence 2014;5:4-11. [Crossref] [PubMed]
- Badiee P, Hashemizadeh Z. Opportunistic invasive fungal infections: diagnosis & clinical management. Indian J Med Res 2014;139:195-204. [PubMed]
- Lepak A, Andes D. Fungal sepsis: optimizing antifungal therapy in the critical care setting. Crit Care Clin 2011;27:123-47. [Crossref] [PubMed]
- Kozel TR, Wickes B. Fungal diagnostics. Cold Spring Harb Perspect Med 2014;4:a019299. [Crossref] [PubMed]
- Sanguinetti M, Posteraro B. Mass spectrometry applications in microbiology beyond microbe identification: progress and potential. Expert Rev Proteomics 2016.1-13. [Epub ahead of print]. [PubMed]
- Florio W, Tavanti A, Barnini S, et al. Recent Advances and Ongoing Challenges in the Diagnosis of Microbial Infections by MALDI-TOF Mass Spectrometry. Front Microbiol 2018;9:1097. [Crossref] [PubMed]
- Hou TY, Wang SH, Liang SX, et al. The Screening Performance of Serum 1,3-Beta-D-Glucan in Patients with Invasive Fungal Diseases: A Meta-Analysis of Prospective Cohort Studies. PLoS One 2015;10:e0131602. [Crossref] [PubMed]
- Mikulska M, Calandra T, Sanguinetti M, et al. The use of mannan antigen and anti-mannan antibodies in the diagnosis of invasive candidiasis: recommendations from the Third European Conference on Infections in Leukemia. Crit Care 2010;14:R222. [Crossref] [PubMed]
- Arvanitis M, Anagnostou T, Fuchs BB, et al. Molecular and nonmolecular diagnostic methods for invasive fungal infections. Clin Microbiol Rev 2014;27:490-526. [Crossref] [PubMed]
- Lippi G. Sepsis biomarkers: past, present and future. Clin Chem Lab Med 2019. [Epub ahead of print]. [PubMed]
- Lippi G, Cervellin G. Procalcitonin for diagnosing and monitoring bacterial infections: for or against? Clin Chem Lab Med 2018;56:1193-5. [Crossref] [PubMed]
- Dornbusch HJ, Strenger V, Kerbl R, et al. Procalcitonin--a marker of invasive fungal infection? Support Care Cancer 2005;13:343-6. [Crossref] [PubMed]
- Beed M, Sherman R, Holden S. Fungal infections and critically ill adults. Continuing Education in Anaesthesia, Critical Care & Pain 2014;14:262-7. [Crossref]
- R Nath S, Jayapalan S, Nair H, et al. Comparative diagnostic test evaluation of serum procalcitonin and C-reactive protein in suspected bloodstream infections in children with cancer. J Med Microbiol 2017;66:622-7. [Crossref] [PubMed]
- Pieralli F, Corbo L, Torrigiani A, et al. Usefulness of procalcitonin in differentiating Candida and bacterial blood stream infections in critically ill septic patients outside the intensive care unit. Intern Emerg Med 2017;12:629-35. [Crossref] [PubMed]
- Thomas-Rüddel DO, Poidinger B, Kott M, et al. Influence of pathogen and focus of infection on procalcitonin values in sepsis patients with bacterial sepsis or candidemia. Crit Care 2018;22:128. [Crossref] [PubMed]
- Chenevier-Gobeaux C, Borderie D, Weiss N, et al. Presepsin (sCD14-ST), an innate immune response marker in sepsis. Clin Chim Acta 2015;450:97-103. [Crossref] [PubMed]
- Yaegashi Y, Shirakawa K, Sato N, et al. Evaluation of a newly identified soluble CD14 subtype as a marker for sepsis. J Infect Chemother 2005;11:234-8. [Crossref] [PubMed]
- Assicot M, Gendrel D, Carsin H, et al. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993;341:515-8. [Crossref] [PubMed]
- Mallet E, Lanse X, Devaux AM, et al. Hypercalcitoninaemia in fulminant meningococcaemia in children. Lancet 1983;1:294. [Crossref] [PubMed]
- Joyce CD, Fiscus RR, Wang X, et al. Calcitonin gene-related peptide levels are elevated in patients with sepsis. Surgery 1990;108:1097-101. [PubMed]
- Bamba Y, Moro H, Aoki N, et al. Increased presepsin levels are associated with the severity of fungal bloodstream infections. PLoS One 2018;13:e0206089. [Crossref] [PubMed]
- Bazhenov A, Galstian GM, Pashkova M, et al. Stimulation of sCD14-ST (Presepsin) Secretion By Peripheral Blood Mononuclear Cells after Candida Albicans Lysate and Lipopolysaccharide Exposure. Blood 2017;130:4811.
- Singer M, Deutschman CS, Warren C, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]


