
Improved long-term survival following pulmonary resections for non-small cell lung cancer: results of a nationwide study from Iceland
Introduction
Lung cancer is a leading cause of cancer-related death in the world. The prognosis remains poor, with only around 13% of patients surviving for 5 years (1). In Europe, the survival of lung cancer patients has gradually increased (1) and this development has also been observed in the Nordic countries (2). Non-small cell lung cancer (NSCLC) accounts for around 80% of lung cancer cases in Europe and Northern America, where pulmonary resection is the treatment of choice for patients diagnosed with stage-I or stage-II disease and for selected patients with stage-IIIA disease (3,4).
Northern-European studies, including one from Iceland, have indicated improved survival following surgical resection for NSCLC (5,6). The reasons for this survival gain remain unclear but advances in the diagnosis of lung cancer, by improving perioperative staging, may have played a role. It is also possible that the use of adjuvant chemotherapy may have increased the survival of surgical patients with stage-II or stage-IIIA disease (7).
Previous studies on surgically treated NSCLC patients have focused mainly on lobectomies, which is the golden standard curative treatment for most patients with disease localized to the lungs (8). However, sublobar resections for patients who are not believed to tolerate lobectomy and pneumonectomies in more advanced disease, still constitute a significant number of patients (9,10). Population-based studies on outcome that include all types of pulmonary resections for NSCLC hardly feature in the literature, with most studies focusing on single or a few specialised centres—with a potential risk of selection bias.
We therefore studied the outcomes of all pulmonary resections for NSCLC in a well-defined population representing the whole of Iceland, during a recent 24-year period, concentrating on long-term survival and identifying prognostic factors for survival.
Methods
Study design, data extraction, and clinical practice
We retrospectively examined all pulmonary resections that were performed with curative intent for primary NSCLC in Iceland from 1 January 1991 until 31 December 2014. Patients with pulmonary metastasis, carcinoid tumours, or recurrent lung cancer; patients who underwent explorative thoracotomy and resection for biopsy purposes; and patients who had resections with advanced or locally advanced disease were excluded. Twenty-one patients who had resections with advanced or locally advanced disease, mostly patients in stage IV at diagnosis due to solitary metastasis in the brain or the adrenal gland, were excluded. Cases were identified using a centralized database at the Department of Pathology, Landspitali University Hospital, covering all surgical specimens of lung cancer. To minimize the risk of missing cases, they were cross-matched with two other independent databases: a diagnostic registry and an operation registry at Landspitali University Hospital.
Landspitali University Hospital is the only hospital in Iceland where cardiothoracic surgery is performed, and this study was therefore performed on a nationwide basis. All surgical procedures were performed under general anaesthesia with double-lumen intubation and thoracic epidural anaesthesia. Between 1991 and 2003, intraoperative lymphadenectomy of enlarged hilar or ipsilateral mediastinal lymph nodes was performed when indicated, but after 2005 these lymph nodes were routinely removed or sampled. Most cases were performed with an anterolateral thoractomy but since 2005, video-assisted thoracic surgery (VATS) was used selectively for sublobar resections.
All patients suspected of having lung cancer were evaluated by a multidisciplinary tumour board including pulmonologists, oncologists, thoracic surgeons, pathologists, and radiologists. The preoperative work-up evolved during the 24-year study period but most commonly included chest radiography; computed tomography (CT) of the chest, upper abdomen, and head; bone scintigraphy; and spirometry. For histological diagnosis, biopsies were obtained through bronchoscopy or transthoracic CT-guided needle biopsy. Mediastinoscopy was performed preoperatively when indicated for cTNM stages Ib to IIIA. Mediastinal staging with endobronchial ultrasound (EBUS) with transbronchial needle aspiration (TBNA) was started in 2014. Furthermore, from 2014, positron emission tomography (PET) scan was performed on selected patients at cTNM stages Ib to III.
All patients were staged postoperatively according to the seventh edition of the TNM classification system. Only pathological staging (pTNM) was consistently available and was therefore used in this analysis. The surgical resection rate represents the ratio of patients undergoing pulmonary resection with histology confirmed NSCLC, according to the Icelandic Cancer Association Registry excluding carcinoid tumours. Incidental diagnosis was defined as no symptoms or signs of NSCLC at the time of diagnosis. Patients who had received adjuvant chemotherapy were identified but no information regarding treatment regimen, adherence, or success was noted. Patients’ date of death was registered, or they were identified as living on 31 December 2017, using data from the Icelandic National Population Registry, which provided almost complete information on long-term survival. The median follow-up time was 45 (mean, 63; range, 1–283) months. To assess trends, we divided the 24-year study period into three 8-year periods.
Statistical analysis
Microsoft Excel 2016 and R software version 3.4.0 were used for data processing and statistical analysis. Chi-squared test was used to compare categorical variables and Fisher’s exact test was used if the values had an expected frequency of 10 or less. Analysis of variance (ANOVA) was used for comparison of continuous variables between two or more groups. The Kaplan-Meier method was used to calculate overall survival and log-rank test was used to compare survival between groups. Any P value of <0.05 was considered to be statistically significant. A Cox proportional-hazards model was used to identify prognostic factors for long-term survival. Factors that had a P value of less than 0.05 in univariate analysis were used in the preliminary model along with factors that have been shown to be significant in other studies. A subset of variables was chosen for inclusion in the final model, using a backwards stepwise selection procedure. Assumption of proportionality was checked using a global goodness-of-fit test together with graphic plotting of variables.
The study was approved by the Icelandic National Bioethics Committee (reference number: 98-060-CM) and the Data Protection Authority (reference number: 2001011025SJ/eb). As individual patients were not identified, the need for individual consent was not required.
Results
Patient demographics, surgical procedures, pathology, and staging
During the 24-year study period, 652 surgical resections were performed on 644 individuals (52% females): 492 lobectomies (75%), 77 pneumonectomies (12%), and 83 sublobar resections (13%) (Table 1). The proportion of pneumonectomies decreased from 13% in 1991−1998 to 7% in 2007−2014 (P=0.005). The surgical resection rate changed from 23% in 1991−1998 to 28% in 2007−2014, but the increase was non-significant (P=0.39).
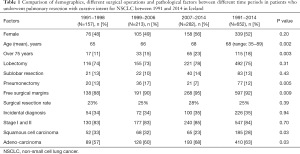
Full table
The mean age of the patients increased from 65 to 68 yrs during the study period (P=0.002) and the proportion of patients over 75 years of age increased from 11% to 23% (P=0.003). The gender ratio, however, did not change significantly; nor did the percentage of patients with ischaemic heart disease. The most common histological types were adenocarcinoma (63%) and squamous cell carcinoma (SCC) (28%), with adenocarcinoma histology increasing significantly (P=0.03) and SCC decreasing significantly (P=0.03) during the study period. There was no significant change in the rate of incidental diagnosis (35%) between time periods (P=0.94).
For the whole study period, the proportions of stage Ia, Ib, IIa, IIb, and IIIA were 30%, 23%, 18%, 13%, and 16%, respectively, as shown in Table 2. The proportion of stage-IA tumours increased from 29% in 1991−1998 to 37% in 2007−2014 (P<0.001).

Full table
Preoperative morbidities and postoperative complications
A comparison of different surgical types is shown in Table 3. A higher proportion of the patients who underwent a sublobar resection had a preoperative diagnosis of IHD (40%), a previous diagnosis of COPD (46%), and a FEV <75% (40%). Major complications were significantly more common in the pneumonectomy group, and a higher proportion of patients had intraoperative bleeding of over 1 L and postoperative new-onset atrial fibrillation. Cancer-free surgical margins (R0-resectons) were also more common in patients who underwent lobectomy (93%) than those who underwent pneumonectomy (87%) or sublobar resection (88%) (P=0.049). Cancer-free surgical margins after pathological examination of the resected lung tissue were found in 88% of the patients between 1991 and 1998 and this increased to 95% in the period 2007−2014 (P=0.009). Eighty-two patients with stage-II or stage-IIIA tumours received adjuvant chemotherapy, most of them in the latest study period (Table S1).
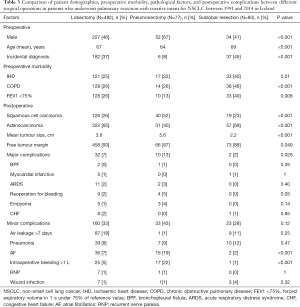
Full table

Full table
Short-term and long-term survival
The postoperative 30- and 90-day mortality, 1.1% and 2.6% respectively, did not change significantly throughout the study period. The 30- and 90-day mortality for pneumonectomies was 5.2% and 9.1%, respectively, and it was 0.6% and 1.8% for lobectomies and 0% and 1.2% sublobar resections.
The overall 5-year survival was 46% and the median survival was 50 (95% CI: 45−61) months. As shown in Table 4, survival increased from 75% to 88% at 1 year postoperatively, from 52% to 66% at 3 years, and from 38% to 53% at 5 years during the study periods. The 5-year overall survival was 50% for lobectomy, 41% for sublobar resection, and 26% for pneumonectomy (P<0.001) (Figure 1A). For the whole period, the 5-year survival for patients with stage-IA tumours and stage-IIIA tumours was 64% and 23%, respectively.

Full table
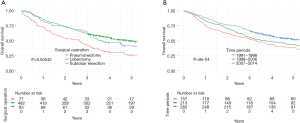
Figure 1B shows the 5-year survival according to study periods. Six months postoperatively, the survival curves started to deviate from each other with more favourable survival observed in the later study periods, both at 1 and 5 years (log-rank test, P<0.001). A subgroup analysis of survival for each stage showed that patients with stage-II disease in the latest time period had significantly improved survival (P=0.015), while patients with stage-I disease (P=0.11) and stage-IIIA disease (P=0.39) did not (Figure S1).

Independent prognostic factors for survival
Figure 2 shows a forest plot with the prognostic factors for overall survival, based on a Cox multivariate analysis. Patients who were operated on in the years 2007−2014 (HR =0.61, 95% CI: 0.48−0.78; P<0.001) and 1999−2006 (HR =0.77, 95% CI: 0.61−0.98; P=0.03) had significantly more favourable survival than patients who were operated on in the period 1991−1998. Patients with stage-IIA, -IIB, and -IIIA disease had significantly worse survival relative to patients with stage-IA disease (HR =2.14, HR =2.21, and HR =2.68, respectively). Advanced age (HR =1.04 per year), a history of ischaemic heart disease (HR =1.26), and a minor postoperative complication (HR =1.26) were significant adverse prognostic factors for overall survival, but free surgical margins were predictive of more favourable survival (HR =0.59). Patients who underwent sublobar resections had worse survival than lobectomy patients (HR =1.33). Adjuvant chemotherapy was not a prognostic factor for survival in univariate (HR =1.12, P=0.46) or multivariate models (HR =0.92, P=0.64).

Discussion
In this nationwide study, we investigated short-term and long-term outcome after all pulmonary resections for NSCLC in a well-defined cohort of patients treated over a 24-year period. We found a significant gain in survival during the study period, with 5-year survival improving from 38% in 1991–1998 to 53% in 2007–2014 (P<0.001). Furthermore, improved outcome in the later time periods was confirmed in a multivariate Cox analysis with a HR of 0.61 for 2007–2014 relative to the first period [1991–1998] (P<0.001).
The explanation for this improvement in long-term survival is most likely multifactorial, but may not be fully accounted for by our data, although some distinctive indicators can be identified. Notably, the proportion of stage-IA patients rose from 29% in 1991−1998 to 37% in 2007−2014 (P<0.001). This is not explained by an increase in incidental detection that did not change during the study period, despite the increased access to imaging with CT in Iceland over this period. Still an increased ratio of incidental diagnosis with CT, and not conventional chest X-ray, as shown in a study from Iceland (11), could have increased the overall survival for the whole study population. Improved imaging techniques and increased public awareness may also have contributed to earlier diagnosis in incidentally diagnosed patients, and thus may have resulted in the improved outcome in later time periods.
Interestingly, the largest proportional gain in survival was seen in patients with stage-II disease. Advances in preoperative diagnostic work-up with more emphasis on mediastinal staging with use of mediastinoscopy, EUS/EBUS, and PET are likely to have improved patient selection for surgery during the study period. These factors are probably mostly relevant in the latest study period [2007−2014], where the most improvement in survival was observed. Furthermore, during the past 15 years in Iceland, ipsilateral mediastinal lymph node dissection has been routinely performed during pulmonary resections. This ensures that patients with stage-II disease and N2 metastasis are staged correctly, and therefore receive adjuvant therapy when indicated. Starting in 2005, a formal multidisciplinary tumour board has discussed all new lung cancer cases in Iceland, formulating treatment plan recommendations. All the factors mentioned above could help decrease the number of understaged patients who receive surgical treatment, but the improved patient selection based on quality diagnostic work-up further ensures that only patients who are truly stage-I, -II, and -IIIA are considered for surgery.
The 1-year survival during the whole study period was 83%, but it was 88% in the latest period, which is comparable to what has been found in other studies (5,12). The 5-year survival for NSCLC after pulmonary resection, which was originally reported as being around 40% (13), has more recently been in the 54−65% range in larger cohorts (12,14-16), but is still around 40% in smaller cohorts (17). In the present study, 5-year survival changed from 38% in 1991−1998 to 53% in 2007−2014. The improved survival in our study is in agreement with recent studies on NSCLC patients (1,18,19), including a Norwegian population-based study (5), that identified resected NSCLC patients as a subgroup of all NSCLC patients with the greatest improvement in survival. Morgant et al. reported on a large study from France (20) and Lee et al. reported on a longitudinal study from South Korea (16), both of which found improved survival in resected NSCLC patients in recent decades. A higher proportion of female patients and stage-IA cases together with a lower number of pneumonectomy cases were identified as possible causes of improved survival in the South Korean study (16). In the present study, adjuvant chemotherapy was not a prognostic factor for survival, and although stage-II and -IIIA patients routinely received adjuvant chemotherapy in the latter half of the study, conclusions regarding the possible impact are difficult to make due to the uncontrolled nature of the study.
Short-term survival was acceptable, with a 30-day mortality of 1.1%, and it did not change substantially over the study period. Other studies have found a 30-day mortality of around 2−4% (21,22) and higher rates for patients who have undergone pneumonectomies, or in the 5−12% range (22,23). In our cohort, the 30-day survival for pneumonectomy patients was 5%, which can be considered acceptable.
Minor complications were a significant adverse prognostic factor for survival in the logistic regression model (HR =1.26). This has been demonstrated previously in the literature (24). The major and minor complication rates were 7% and 33% respectively; the latter being significantly higher in 1999−2006 than in the other periods. It could have played a part in improved survival in the latest time period, but there was no clear development over the whole study period. The complication rate after lobectomies has been reported to range between 19% and 58% (24,25), which is in line with our findings. As expected, patients who underwent pneumonectomy had higher minor and major complication rates, of 43% and 13%, respectively, which correlate with increased postoperative morbidity in this patient group.
The ratio of cancer-free surgical margins (R0 resections) increased over the study period, and surgical margins free of tumour growth were an independent prognostic factor for increased survival. The dismal effect of R1 pulmonary resections on survival is previously well established (26).
The main strength of this study was the low risk of selection bias, as it included all the patients who underwent pulmonary resection for NSCLC over 24 years in a whole nation. The main limitation of the study was its retrospective design, with the potential bias that it can introduce, such as missing information on symptoms, lack of complete preoperative staging, and lack of documentation of complications. The lack of detailed information regarding the use of adjuvant chemotherapy limited the possibility of careful assessment of its significance in this study.
In conclusion, the survival of patients operated for NSCLC has improved significantly in Iceland. This improvement in survival may be explained by an increased number of patients being diagnosed at lower stages. Improved preoperative staging, resulting in fewer understaged patients who get selected for a surgical treatment, may have contributed to this increase in survival—but we found no evidence that adjuvant chemotherapy for stage-II and stage-IIIA patients significantly affected survival.
Acknowledgements
We thank the members of the Lung Cancer Research Group at Landspitali University Hospital for help with data collection, the Statistical Consulting Centre at the School of Health Sciences, University of Iceland, for help with statistical analysis, and Gunnhildur Johannsdottir for secretarial help. This study was supported by grants from Landspitali University Research Fund and the University of Iceland Research Fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Icelandic National Bioethics Committee (reference number: 98-060-CM) and the Data Protection Authority (reference number: 2001011025SJ/eb). As individual patients were not identified, the need for individual consent was not required.
References
- De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE--5-a population-based study. Lancet Oncol 2014;15:23-34. [Crossref] [PubMed]
- Engholm G, Ferlay J, Christensen N, et al. NORDCAN--a Nordic tool for cancer information, planning, quality control and research. Acta Oncol 2010;49:725-36. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Baldvinsson K, Oskarsdottir GN, Orrason AW, et al. Resection rate and operability of elderly patients with non-small cell lung cancer: Nationwide study from 1991 to 2014. Interact Cardiovasc Thorac Surg 2017;24:733-9. [Crossref] [PubMed]
- Nilssen Y, Strand TE, Fjellbirkeland L, et al. Lung cancer survival in Norway, 1997-2011: from nihilism to optimism. Eur Respir J 2016;47:275-87. [Crossref] [PubMed]
- Oskarsdottir GN, Halldorsson H, Sigurdsson MI, et al. Lobectomy for non-small cell lung carcinoma: a nationwide study of short- and long-term survival. Acta Oncol 2017;56:936-42. [Crossref] [PubMed]
- Burdett S, Pignon JP, Tierney J, et al. Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database Syst Rev 2015.CD011430. [PubMed]
- Vansteenkiste J, Crino L, Dooms C, et al. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol 2014;25:1462-74. [Crossref] [PubMed]
- Deng HY, Wang YC, Ni PZ, et al. Radiotherapy, lobectomy or sublobar resection? A meta-analysis of the choices for treating stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2017;51:203-10. [PubMed]
- Donington JS, Pass HI. Surgical approach to locally advanced non-small cell lung cancer. Cancer J 2013;19:217-21. [Crossref] [PubMed]
- Orrason AW, Sigurdsson MI, Baldvinsson K, et al. Incidental detection by computed tomography is an independent prognostic factor for survival in patients operated for nonsmall cell lung carcinoma. ERJ Open Research 2017;3:00106-2016. [Crossref] [PubMed]
- Voltolini L, Bongiolatti S, Luzzi L, et al. Impact of interstitial lung disease on short-term and long-term survival of patients undergoing surgery for non-small-cell lung cancer: analysis of risk factors. Eur J Cardiothorac Surg 2013;43:e17-23. [Crossref] [PubMed]
- van Rens MT, de la Riviere AB, Elbers HR, et al. Prognostic assessment of 2,361 patients who underwent pulmonary resection for non-small cell lung cancer, stage I, II, and IIIA. Chest 2000;117:374-9. [Crossref] [PubMed]
- Backhus LM, Farjah F, Liang CK, et al. Imaging surveillance and survival for surgically resected non-small-cell lung cancer. J Surg Res 2016;200:171-6. [Crossref] [PubMed]
- Takenaka T, Katsura M, Shikada Y, et al. The impact of cardiovascular comorbidities on the outcome of surgery for non-small-cell lung cancer. Interact Cardiovasc Thorac Surg 2013;16:270-4. [Crossref] [PubMed]
- Lee JG, Lee CY, Bae MK, et al. Changes in the Demographics and Prognoses of Patients with Resected Non-Small Cell Lung Cancer: A 20-Year Experience at a Single Institution in Korea. J Korean Med Sci 2012;27:1486-90. [Crossref] [PubMed]
- Wright GM, Thursfield VJ, Ball DL, et al. Surgical resection and long-term survival outcome for non-small cell lung cancer: a comparison of Victorian population-based studies spanning a decade. Asia Pac J Clin Oncol 2014;10:75-9. [Crossref] [PubMed]
- Subramanian J, Morgensztern D, Goodgame B, et al. Distinctive characteristics of non-small cell lung cancer (NSCLC) in the young: a surveillance, epidemiology, and end results (SEER) analysis. J Thorac Oncol 2010;5:23-8. [Crossref] [PubMed]
- Lara MS, Brunson A, Wun T, et al. Predictors of survival for younger patients less than 50 years of age with non-small cell lung cancer (NSCLC): a California Cancer Registry analysis. Lung Cancer 2014;85:264-9. [Crossref] [PubMed]
- Morgant MC, Pages PB, Orsini B, et al. Time trends in surgery for lung cancer in France from 2005 to 2012: a nationwide study. Eur Respir J 2015;46:1131-9. [Crossref] [PubMed]
- Kozower BD, Sheng S, O'Brien SM, et al. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg 2010;90:875-81; discussion 881-3. [Crossref] [PubMed]
- Harpole DH Jr, DeCamp MM Jr, Daley J, et al. Prognostic models of thirty-day mortality and morbidity after major pulmonary resection. J Thorac Cardiovasc Surg 1999;117:969-79. [Crossref] [PubMed]
- Stolz AJ, Harustiak T, Simonek J, et al. Pneumonectomy for non-small cell lung cancer: predictors of early mortality and morbidity. Acta Chir Belg 2014;114:25-30. [Crossref] [PubMed]
- Andalib A, Ramana-Kumar AV, Bartlett G, et al. Influence of postoperative infectious complications on long-term survival of lung cancer patients: a population-based cohort study. J Thorac Oncol 2013;8:554-61. [Crossref] [PubMed]
- Irie M, Nakanishi R, Yasuda M, et al. Risk factors for short-term outcomes after thoracoscopic lobectomy for lung cancer. Eur Respir J 2016;48:495-503. [Crossref] [PubMed]
- Wind J, Smit EJ, Senan S, et al. Residual disease at the bronchial stump after curative resection for lung cancer. Eur J Cardiothorac Surg 2007;32:29-34. [Crossref] [PubMed]


