Safety and efficacy of computed tomography-guided dye localization using patent blue V for single lung nodule for video-assisted thoracoscopic surgery: a retrospective study
Introduction
In 2017, lung cancer was one of the leading causes of cancer-related mortality in the United States, accounting for up to 26% cases (1). The National Lung Screening Trial Research Team reported that the use of low-dose computed tomography (LDCT) as a screening tool to detect early-stage lung cancer could reduce mortality up to 20% among smokers or ex-smokers (2). Compared with chest radiography and conventional CT, LDCT provided more detailed information about lung disease with less radiation dose. Of note, patients experiencing symptoms such as cough, hemoptysis, dyspnea, or chest pain and high-risk patients, such as smokers or those with a cancer history, are more likely to undergo CT scanning. Reportedly, the incidental pulmonary nodules could be classified as benign and malignant, such as lung cancer, metastases, carcinoid tumor, infectious granuloma, or benign tumor (3). If nodules exhibit suspicious image features or are indeterminate, nonsurgical biopsy or excision could be performed for the tissue diagnosis.
Surgical excision is the gold standard for diagnosis among the management options for cancer, also providing other benefits, such as diagnosis, tumor staging, lymph node sampling, and treatment within a single operative procedure. Additionally, minimally invasive thoracic surgery (MITS), including video- or robotic-assisted thoracoscopic surgery, offers advantages such as faster recovery time, shorter hospitalization, reduced postoperative pain, and smaller chest wall incision, making it a favorite option for surgeons compared with open thoracotomy (4-6). Nevertheless, MITS has some limitations. Tsai et al. reported the challenges in recognizing small, subsolid, or deeply-seated pulmonary nodules during video-assisted thoracoscopic surgery (VATS) (7); these pulmonary nodules were either invisible or impalpable. Previously, several localization methods have been developed to enhance the accuracy of resection; however, those methods with different localized material or modality exhibited variable complications, advantages, or drawbacks (8). For instance, the dislodgement rate was about 2.4–6.9% at the hookwire localization. Hence, this study aims to assess the safety and efficacy of the preoperative computed tomography (CT)-guided localization with patent blue V dye.
Methods
Study design and patients
In this retrospective study, we enrolled patients who had a lung nodule and underwent the preoperative CT-guided patent localization from March 2013 to March 2015. We obtained informed consent from all patients before the procedure. Of note, we excluded patients who had undergone other methods of localization (Figure 1).
Study procedure
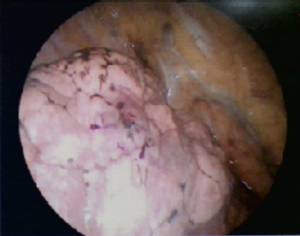
In this study, dye localization was performed (Figure 2) under a 4- or 16-slice CT (HiSpeed CT/i or GE LightSpeed; GE Healthcare, Milwaukee, WI, USA). After confirming the location of the lung nodule with a surgeon, we planned a needle tract as short as possible to avoid transverse through a large vessel, bulla, or interlobar fissure. A 22-gage, 15-cm Chiba needle (PTC needle, Hakko, Nagano, Japan) was inserted into the target nodule under CT scan after sterilization and local anesthesia with 2% lidocaine. Next, 0.1–0.2 mL dye (patent blue V 2.5%; Guerbet, Aulnay-sous-Bois, France) was administered through a needle into the target lesion. If the lesion was far from the pleura, the dye was injected multiple times along the tract between the nodule and the subpleural region. We arranged the final CT to validate the result of the dye localization and postprocedural complication, including pneumothorax or hemorrhage. Then, patients were transferred to the operating room/ward for VATS, where a surgeon could resect the lesion along the trace of dye (Figure 3).
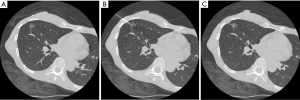
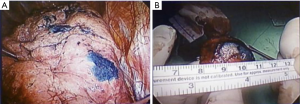
To simplify our comparison, we excluded patients with >1 nodule to preoperative localization or those who underwent additional procedure during operation from our study (Figure 1). We analyzed the image features of lung nodules, including the nodule size, attenuation, and distance to the nearest visceral pleural or fissure surface. Furthermore, we analyzed the details of surgery and localization, including the distance of needle transversing the lungs, procedural time, postprocedural complication, and postoperative hospital stay.
Results
In this study, we enrolled 518 nodules of 430 patients who had a lung nodule and underwent the preoperative CT-guided patent localization from March 2013 to March 2015. Of these, 422 patients underwent the dye localization with patent blue V, while the other 8 underwent the preoperative localization with patent dye blue V and hookwire owing to multiple lung nodules. Of the remaining patients, 66 patients with >1 nodule to the preoperative localization were excluded from the analysis. Furthermore, 74 patients with additional intraoperative procedure were excluded. Hence, we finally analyzed 282 patients in this study.
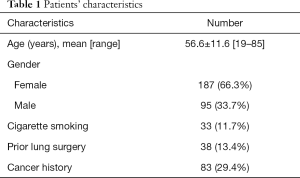
Table 1 summarizes the patients’ demographics. The mean age of our study sample was 56.6±11.6 (range, 19–85) years, with female preponderance (male/female, 95/187). Of all, 33 patients were either smokers or ex-smokers, and 83 had a cancer history. Furthermore, 38 patients had undergone lung surgery in the past, and CT revealed visible emphysema in 4 patients.
Full table
Table 2 presents the image features of nodules. The mean size of nodules was 0.9±0.5 (range, 0.3–3.2) cm. There were 67 solid nodules, 60 subsolid nodules, 151 pure ground-glass nodule, and 4 nodules with cavitation. In 41 patients, the nodule depth from the visceral pleural or interlobar fissure surface was >2 cm.
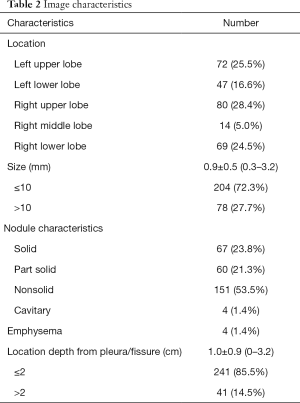
Full table
Table 3 presents each parameter of localization such as the depth of the needle transversing the lungs, procedural time, and complication rate. The mean depth of the needle transversing the lungs was 1.9±1.2 cm. Overall, 15 patients had a deep needle penetrating route with >4 cm length. The mean localization duration was 24 (range, 3–70) min. Except for two cases with the poor or fail dye localization, our localization procedures were performed successfully with minimal or acceptable complications; these two cases underwent successful resection after cautious palpation. In addition, four cases used two Chiba needles during the localization because the tip of the first needle was far away from the target lesion, whereas three cases reported the transfissural route of localization. In our localization, the leading complications were pneumothorax (48 patients, 17%) and mild pulmonary hemorrhage (51 patients, 18%); all were asymptomatic under the oxygen supplement through a nasal cannula. Other rare complications were subcutaneous hematoma or subcutaneous emphysema, which spontaneously resolved without treatment.
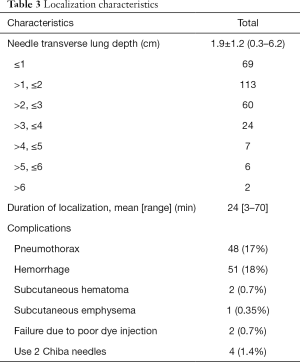
Full table
Table 4 presents the type of surgery and pathological results. In this study, most patients underwent wedge resection (78.3%) and segmentectomy (12.8%); the remaining patients received lobectomy after intraoperative frozen-proven malignancy (25 patients, 8.9%). The mean duration of lung operation was 105 (range, 35–325) min. In addition, the leading pathology in this study was lung cancer and premalignant lesions (74.1%). The ratio of the benign nodule was about 19.1%. Residual nodules include metastases or lymphoma.
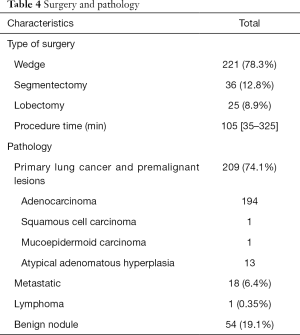
Full table
Table 5 summarizes the details of patients’ outcomes. Of note, three patients reported air leak and one experienced chylothorax, receiving pleurodesis postoperatively. Additionally, one patient had empyema, receiving decortication and empiric antibiotics during readmission; one patient had postoperative left-side weakness, and the brain magnetic resonance imaging (MRI) revealed with right middle cerebral artery infarction. We noted no definite image evidence of embolic infarction in the brain MRI. Notably, one patient died due to the left ventricular rupture, probably because of acute myocardial infarction.
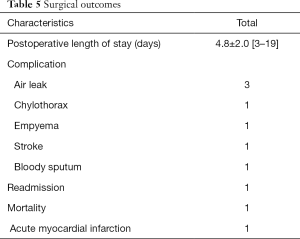
Full table
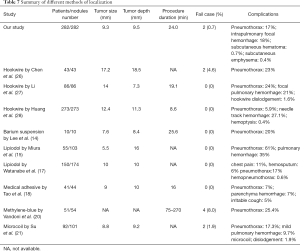
VATS is a minimally invasive procedure, which provides short recovery time and one-step diagnosis and treatment; hence, most surgeons preferred this procedure for treating indeterminate lung nodules. However, a study has reported small subsolid characteristics of VATS (9). Previously, several localization methods have been developed to enhance the accuracy of resection; however, those methods with different localized material or modality exhibited variable complications, advantages, or drawbacks. Arguably, hookwire localization is a safe and effective method; however, its limitation is dislodgement. Reportedly, the nodules near the hilum or scapula are also not suitable for the hookwire localization (10,11). In some studies, the intraoperative ultrasonography was highly operator-dependent and was not suitable for pure ground-glass nodule or lung(s) with emphysema (12,13). Some liquid contrast media, such as lipiodol or barium, have been applied for localization; however, they amplified the radiation exposure to surgeons, increasing the likelihood of errors in pathological results owing to the focal inflammation of specimens (14-17). Furthermore, some studies have provided details of other methods or materials, such as isotope, medical adhesive, methylene blue dye, microcoil, or electromagnetic navigation bronchoscopy (18-23) (Tables 6,7).

Full table
Full table
Patent blue V, a synthetic triphenylmethane dye, has been extensively used in sentinel lymph node mapping in patients with malignancy. Haque et al. reported six patients with an allergic reaction, such as hypotension, bronchoconstriction, erythema, urticaria, and angioedema, to patent blue V during sentinel lymph node mapping for breast cancer (29). Likewise, Mertes reported 14 cases of hypersensitivity reaction between 2004 and 2006; 6 patients had cardiovascular collapse (30). In this study, we noted no associated allergic reactions.
This study revealed that the CT-guided dye localization was a useful method to aid sublobar resection of lung nodules. Previously, we faced challenges in dealing with deep lung nodules owing to nonvisualization of the dye at the surface of the lung parenchyma. In this study, we injected the dye from the lung nodule to the subpleural region of the lungs when pulling the Chiba needle backward stepwise. The surgical outcome revealed that the CT-guided dye localization is a feasible technique for the preoperative localization of small deep lung nodules. The surgeon found the lung nodule along the trace of the dye at the lung surface, and the excision of the lung nodule at VATS could be performed smoothly. Moreover, the surgeon could make decisions about the need for additional surgery based on the results of frozen histopathology. Furthermore, the CT-guided dye localization could help avoid needless lobectomy.
Compared with other methods of localization, the advantage of the dye is the simplicity by using a 22-gauge Chiba needle and CT equipment, eliminating any need of receiving additional radiation dose for an operator during the procedure. In fact, multiple nodules localization could also be manipulated by the CT-guided dye localization simultaneously. In our procedure, the common complications were pneumothorax and focal lung hemorrhage; most patients were asymptomatic and only dealing with nasal cannula oxygenation. Few patients needed the insertion of a chest tube to drainage the air. In this study, two patients required insertion of the second Chiba needle because of malposition of the first Chiba needle with moderate pneumothorax. Choosing the small-diameter needle, avoiding transversing the pleura recurrently, and the transfissural route could minimize the risk of pneumothorax or other complications. We did not observe rare complications or an anaphylactic reaction in our patients.
The disadvantages of the dye localization are poor visualization of the dye in severely anthracotic or pigmented lung. Other limitations of the dye localization are gradual absorbance and diffusion. Thus, the operation at the same day of localization is the ideal condition.
In this study, two cases exhibited nonvisualization or poor identification of the dye during VATS. The first case (Figures 4,5) with mild pneumothorax during the procedure and shallow needle insertion depth was found at the image; this could be attributed to the dislodgement of the needle into the pleural cavity during the dye injection. The injected dye was mixed with the pleural fluid and became invisible to the surgeon during VATS. The second case (Figure 6) had a deeper lung nodule at the image. The localization review revealed that the final site of the dye injection was far away from the subpleural region; the distance between the lung surface and the needle tip was >1 cm. Perhaps, the poor dye visualization at the lung surface was caused by the remote distance; the dye was injected too deep to be viewed from the lung surface. Other causes of failed dye localization might include moderate or severe pneumothorax or uncooperative patients, making the lesion unapproachable or prolong the procedure duration during the localization.
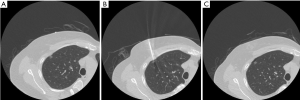

This study has several limitations. First, it is a retrospective study conducted in a single center. Second, other variable factors could have affected the outcome of patients, including different surgical methods or underlying medical condition of patients. Third, we found few cases with other localization techniques at our institution, making it challenging to compare the pros and cons of different localization methods in this study. Finally, the study only reviewed patients with a single lung nodule. Hence, comprehensive studies with larger sample size are warranted.
Conclusions
This study reveals that the preoperative CT-guided dye localization of small deep lung lesion is a safe and effective method with acceptable mild complications and high success rate, making manipulating the sublobar resection smooth.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was performed in accordance with the ethical standards detailed in the Declaration of Helsinki. All patients have provided written informed consent.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7. [Crossref] [PubMed]
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Rubins JB, Rubins HB. Temporal trends in the prevalence of malignancy in resected solitary pulmonary lesions. Chest 1996;109:100. [Crossref] [PubMed]
- Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362. [Crossref] [PubMed]
- Kirby TJ, Mack MJ, Landreneau RJ, et al. Lobectomy-video-assisted thoracic surgery versus muscle-sparing thoracotomy. A randomized trial. J Thorac Cardiovasc Surg 1995;109:997. [Crossref] [PubMed]
- Darr C, Cheufou D, Weinreich G, et al. Robotic thoracic surgery results in shorter hospital stay and lower postoperative pain compared to open thoracotomy: a matched pairs analysis. Surg Endosc 2017;31:4126. [Crossref] [PubMed]
- Tsai TM, Hung WT, Lin MW, et al. Computed tomography-guided dye localization prior to uniportal thoracoscopic surgery for lung nodules: A propensity score matching analysis. J Formos Med Assoc 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Lin MW, Chen JS. Image-guided techniques for localizing pulmonary nodules in thoracoscopic surgery. J Thorac Dis 2016;8:S749-55. [Crossref] [PubMed]
- Kim MP, Nguyen DT, Chan EY, et al. Computed tomography criteria for the use of advanced localization techniques in minimally invasive thoracoscopic lung resection. J Thorac Dis 2018;10:3390-8. [Crossref] [PubMed]
- Gonfiotti A, Davini F, Vaggelli L, et al. Thoracoscopic localization techniques for patients with solitary pulmonary nodule: hookwire versus radio-guided surgery. Eur J Cardiothorac Surg 2007;32:843-7. [Crossref] [PubMed]
- Klinkenberg TJ, Dinjens L, Wolf RF, et al. CT-guided percutaneous hookwire localization increases the efficacy and safety of VATS for pulmonary nodules. J Surg Oncol 2017;115:898-904. [Crossref] [PubMed]
- Matsumoto S, Hirata T, Ogawa E, et al. Ultrasonographic evaluation of small nodules in the peripheral lung during video-assisted thoracic surgery (VATS). Eur J Cardiothorac Surg 2004;26:469-73. [Crossref] [PubMed]
- Kondo R, Yoshida K, Hamanaka K, et al. Intraoperative ultrasonographic localization of pulmonary ground-glass opacities. J Thorac Cardiovasc Surg 2009;138:837-42. [Crossref] [PubMed]
- Lee NK, Park CM, Kang CH, et al. CT-guided percutaneous transthoracic localization of pulmonary nodules prior to video-assisted thoracoscopic surgery using barium suspension. Korean J Radiol 2012;13:694-701. [Crossref] [PubMed]
- Miura H, Yamagami T, Tanaka O, et al. CT findings after lipiodol marking performed before video-assisted thoracoscopic surgery for small pulmonary nodules. Acta Radiol 2016;57:303-10. [Crossref] [PubMed]
- Hu M, Zhi X, Zhang J. Preoperative computed tomography-guided percutaneous localization of ground glass pulmonary opacity with polylactic acid injection. Thoracic Cancer 2015;6:553-6. [Crossref] [PubMed]
- Watanabe K, Nomori H, Ohtsuka T, et al. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg 2006;132:320-4. [Crossref] [PubMed]
- Tao G, Jingying Y, Tan G, et al. A novel CT-guided technique using medical adhesive for localization of small pulmonary ground-glass nodules and mixed ground-glass nodules (≤20 mm) before video-assisted thoracoscopic surgery. Diagn Interv Radiol 2018;24:209-12. [Crossref] [PubMed]
- Petsas T, Siamblis D, Giannakenas C, et al. Fibrin glue for sealing the needle track in fine-needle percutaneous lung biopsy using a coaxial system: part II-Clinical study. Cardiovasc Intervent Radiol 1995;18:378-82. [Crossref] [PubMed]
- Vandoni RE, Cuttat JF, Wicky S, et al. CT-guided methylene-blue labeling before thoracoscopic resection of pulmonary nodules. Eur J Cardiothorac Surg 1998;14:265-70. [Crossref] [PubMed]
- Su TH, Fan YF, Jin L, et al. CT-guided localization of small pulmonary nodules using adjacent microcoil implantation prior to video-assisted thoracoscopic surgical resection. Eur Radiol 2015;25:2627-33. [Crossref] [PubMed]
- Bolton WD, Howe H III, Stephenson JE. The utility of electromagnetic navigational bronchoscopy as a localization tool for robotic resection of small pulmonary nodules. Ann Thorac Surg 2014;98:471-5. [Crossref] [PubMed]
- Anantham D, Feller-Kopman D, Shanmugham LN, et al. Electromagnetic navigation bronchoscopy-guided fiducial placement for robotic stereotactic radiosurgery of lung tumors: A feasibility study. Chest 2007;132:930-5. [Crossref] [PubMed]
- Lin MW, Tseng YH, Lee YF, et al. Computed tomography-guided patent blue vital dye localization of pulmonary nodules in uniportal thoracoscopy. J Thorac Cardiovasc Surg 2016;152:535-44.e2. [Crossref] [PubMed]
- Tseng YH, Lee YF, Hsieh MS, et al. Preoperative computed tomography-guided dye injection to localize multiple lung nodules for video-assisted thoracoscopic surgery. J Thorac Dis 2016;8:S666-71. [Crossref] [PubMed]
- Chen S, Zhou J, Zhang J, et al. Video-assisted thoracoscopic solitary pulmonary nodule resection after CT-guided hookwire localization: 43 cases report and literature review. Surg Endosc 2011;25:1723-9. [Crossref] [PubMed]
- Li C, Liu B, Jia H, et al. Computed tomography-guided hook wire localization facilitates video-assisted thoracoscopic surgery of pulmonary ground-glass nodules. Thorac Cancer 2018;9:1145-50. [Crossref] [PubMed]
- Huang HZ, Wang GZ, Xu LC, et al. CT-guided Hookwire localization before video-assisted thoracoscopic surgery for solitary ground-glass opacity dominant pulmonary nodules: radiologic-pathologic analysis. Oncotarget 2017;8:108118-29. [Crossref] [PubMed]
- Haque RA, Wagner A, Whisken JA, et al. Anaphylaxis to patent blue V: a case series and proposed diagnostic protocol. Allergy 2010;65:396. [Crossref] [PubMed]
- Mertes PM, Malinovsky JM, Mouton-Faivre C, et al. Anaphylaxis to dyes during the perioperative period: reports of 14 clinical cases. J Allergy Clin Immunol 2008;122:348. [Crossref] [PubMed]