Predictive risk factors of acute kidney injury after on-pump coronary artery bypass grafting
Introduction
Acute kidney injury (AKI) is a common complication after coronary artery bypass grafting (CABG) and increases the risk of short and long-term morbidity and mortality (1-4). Actually, the incidence of AKI after cardiac surgery is from 1% to 30% (1,5). In addition, AKI is associated with increased in-hospital mortality and a risk of progression to chronic kidney disease (CKD) (6). After cardiac surgery, renal replacement therapy (RRT) requiring AKI leads to a mortality rate as high as 25% (7,8). Moreover, even small elevation of postoperative serum creatinine (s-Cr) level causes significant adverse outcomes (1,2,9). In fact, patients with mild AKI are usually responsive to medical therapy and eventually show spontaneous recovery. Clinicians can use predictive risk factors to better stratify the risk for AKI in patients undergoing cardiac surgery and to help inform their decision to operate. Therefore, prediction of AKI is very important in both physicians and surgeons. The aim of study is to investigate preoperative and intraoperative risk factors for development of AKI after primary isolated on-pump CABG.
Methods
Patients
This retrospective cohort study included 210 consecutive patients who underwent primary isolated on-pump CABG, from January 2007 to March 2016, at the Yeungnam University Hospital. All patients are Asian race. Patients were excluded from the analysis if they were undergoing RRT before operation, had end-stage renal disease (ESRD). The patients were divided into without AKI group (Group 1) and AKI group (Group 2) after operation.
Collection and definitions
The clinical data of all patients were collected from electronic records. The body surface area (BSA) was calculated by Mosteller formula. The preoperative s-Cr values were defined as within 5 days before the surgery. Postoperative AKI was defined and classified as increase in the s-Cr by 0.3 mg/dL or more or 1.5 times or greater than baseline level, according to the Kidney Disease Improving Global Outcomes (KDIGO) guideline (Table 1). The s-Cr levels were recorded pre and postoperatively (Figure 1).

Full table
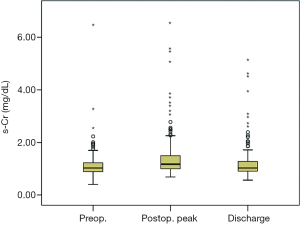
We compared the peak levels of s-Cr between pre-operation and post-operation. Proteinuria was measured with a dipstick test before surgery. Proteinuria was defined as a trace or more of protein on a urine dipstick test. We statistically analyzed variables and characteristics of all patients as risk factors of AKI after surgery. We investigated the correlating factors for the ratio of increase of s-Cr level.
Statistical analysis
Statistical analysis was conducted using the SPSS software package (ver. 23.0, IBM SPSS). Continuous variables were presented as the mean ± standard deviation and categorical variables as a percentage. In order to identify risk factors for AKI we analyzed differences between Group 1 and Group 2.
An unpaired t-test was used to compare the means between two groups, and the chi-squared test was applied to compare the proportions between two groups of subjects. Univariate logistic regression was used to evaluate the risk factors associated with AKI. Multiple logistic regression analysis was performed to identify independent risk factors for AKI.
The data were listed as the odd ratios (ORs) with 95% confidence intervals (CIs). A two-tailed P value <0.05 was considered statistically significant. Linear regression analysis was used to investigate the correlation between risk factors and the ratio of increase of s-Cr level.
Results
Patient characteristics
AKI developed in 40.5% of the patients (85 patients out of 210 patients). We classified AKI into stage 1, 2, and 3 according to KDIGO guideline. Out of 85 patients who developed AKI, 69 patients had stage 1, 12 patients in stage 2, and 4 patients with stage 3. RRT was required to 1 patient in stage 1, 3 patients in stage 2, and 2 patients in stage 3 respectively. The mortality rate of Group 1 was 0.8% (1 patient out of 125 patients) while Group 2 showed the mortality rate of 11.8% (10 patients out of 85 patients) (Table 2).

Full table
Multivariate analysis for predictors of AKI after CABG
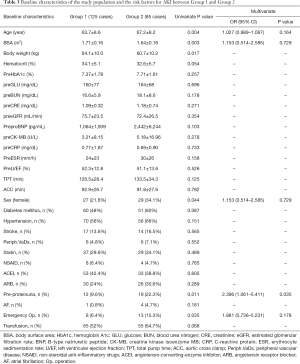
Age (Group 1; Group 2, 63.7±8.6; 67.2±8.2, P=0.004), BSA (Group 1; Group 2, 1.71±0.16; 1.64±0.16, P=0.003), body weight (Group 1; Group 2, 64.1±10.0; 60.7±10.2, P=0.017) were statistically significant between groups. Preoperative hemoglobin, blood urea nitrogen (BUN), creatinine, estimated glomerular filtration rate (eGFR) and C-reactive protein (CRP) were not significant (Table 3). Female gender (OR 1.88; P=0.044, CI: 1.013–3.489), preoperative proteinuria (OR 2.711; P=0.011, CI: 1.238–5.937) and emergent operation (OR 2.641; P=0.035, CI: 1.044–6.682) were risk factors in univariate analysis (Table 3). Diabetes mellitus, hypertension, medication of angiotensin-converting-enzyme inhibitor (ACEI) and angiotensin receptor blocker (ARB) were not statistically significant. As intraoperative factors, total pump time (TPT), aortic cross clamp (ACC) time and transfusion were not statistically significant. For multivariate analysis of preoperative risk evaluation, logistic regression was repeated with variables that had been significant in previous univariate analysis (age, BSA, sex, pre-proteinuria and emergency operation). The backward stepwise multiple logistic regression model revealed that preoperative proteinuria (OR 2.396; P=0.035) was a risk factor in multivariate analysis (Hosmer-Lemeshow’s goodness-of-fit; P=0.620) (Table 3).
Full table
Correlation between the ratio of increase of s-Cr level and parameters
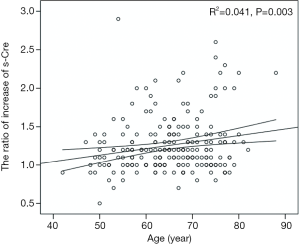
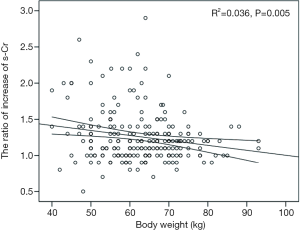
The postoperative peak s-Cr level was increased 1.26±0.36 times than preoperative. Our study showed that emergency operation and transfusion were risk factors of the ratio of increase of s-Cr (Tables 4,5). And age was related to positive correlation in the ratio of increase of s-Cr (Figure 2). In contrast, BSA and body weight were related to negative correlation (Figures 3,4). Age and body weight were processed by the multiple linear regression due to strong linear correlation between body weight and BSA.
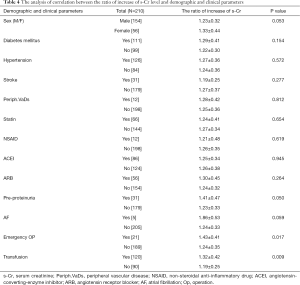
Full table

Full table
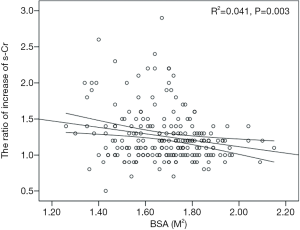
The regression model was established as follows:
The ratio of increase of s-Cr =1.147 + 0.006Age − 0.005Weight (P=0.002).
All of the regression coefficients were acquired, and the significance of each coefficient were 0.041 (age), 0.066 (weight) respectively. Age indicated a valid factor in the regression equation.
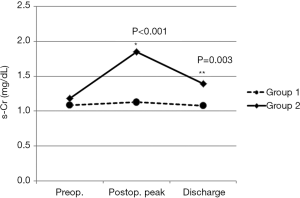
The s-Cr level tends to go up just after the surgery but comes back down shortly after (Figure 1). Also, the s-Cr level in Group 2 was statistically higher than Group 1 on the day of discharge (Figure 5).
Discussion
AKI is an abrupt loss of the kidney function characterized by an acute increase in s-Cr concentration (10). Cardio-renal syndrome is well known interdependency of cardiac and renal dysfunction in heart failure. More recently, growing awareness that heart failure, renal impairment, and anemia are frequent co-morbidities which can exacerbate one another in a vicious circle of clinical deterioration has led to the concept of cardio-renal anemia syndrome.
Postoperative AKI is associated with increased in-hospital mortality and with a significantly increased risk of 5-year readmission and 5-year mortality after cardiac surgery (11). Brown and colleagues (12) had found high rates of readmission within 30 days after cardiac surgery in patients who developed AKI. Cardiac surgery-associated acute kidney injury (CSA-AKI) is a common complication and is associated with increased short and long-term mortality (2,13,14). CSA-AKI occurs in approximately 4% to 9%, with 2% to 6% of patients ultimately developing the need for RRT (14-21). Patients who developed AKI after cardiac surgery are less likely to survive for a long term, regardless of their need for RRT (11). However, the incidence of AKI in patients with isolated CABG is from 12% to 48.5%, (13,15,22-24) and those who developed AKI are associated with mortality of 12.6% (24). Dialysis-requiring AKI in the immediate postoperative period is an independent risk factor for mortality (25). Until now, no pharmacologic interventions have conclusively proved the efficacy in the treatment of AKI after cardiac operation. Recent studies have improved our understanding of the pathogenesis of AKI, but this has not innovatively changed the clinical treatments (26). Meanwhile, early detection of these patients may contribute to improving their outcomes (13,26,27). Clinically, the accurate risk prediction of AKI can help clinicians identify high risk patients for more effective prevention and treatment. The focuses of clinicians are on prevention and management of the preoperative risk factors. Therefore, we evaluated the incidence of AKI and risk factors in the development of AKI. We also identified the contributing factors to the ratio of increase of postoperative s-Cr.
The pathogenesis of CSA-AKI can be divided into preoperative, intraoperative, and postoperative events. CSA-AKI is manifested as a rise in s-Cr and finally leads to a decreased urine output. Our results revealed that the incidence of AKI after surgery was 40.5% with an associated mortality of 11.8%. In the AKI group, the rate of requiring RRT was 7%. Our results demonstrated that older age, BSA, body weight, female gender, preoperative proteinuria and emergent operation were associated with the development of AKI. Among these, only preoperative proteinuria was an independent risk factor.
The postoperative peak s-Cr level was increased 1.26±0.36 times than preoperative. Our study showed that emergency operation and transfusion were risk factors of the ratio of increase of s-Cr. And age was related to positive correlation in the ratio of increase of s-Cr (R=0.202, P=0.003). In contrast, BSA and body weight were related to negative correlation (R=−0.203, P=0.003; R=−0.192, P=0.005). Lassnigg and colleagues (2) have shown that even small increase in creatinine as small as 0.3 mg/dL is associated with increased morbidity and mortality. Therefore, small changes in s-Cr are important and should not be ignored. Moreover, Liotta and colleagues (28) reported that even a minimal elevation in the postoperative s-Cr values of <0.3 mg/dL was associated with increased long-term mortality over 6 years of follow-up, but not with mortality within 30 days of surgery. Therefore, postoperative s-Cr is a powerful predictor of long-term mortality after cardiac surgery (29). We found the s-Cr level in Group 2 was statistically higher than Group 1 on the day of discharge. We need to follow up the s-Cr level in AKI group.
The reasons why small changes in s-Cr correlated with increased hospital mortality are not entirely clear. We can explain in the following reasons, the adverse effects of AKI, such as volume overload, uremia, metabolic acidosis, electrolyte disturbances, and increased risk of infections (30,31). Elevated s-Cr may be associated with increased morbidity and mortality even when its change does not meet the criteria for AKI (32). Ho and colleagues (33) showed that measurement of s-Cr, within 6 hours of completion of cardiac surgery predicted AKI significantly.
Novel biomarkers, such as neutrophil gelatinase-associated lipocalin and cystatin C, have been correlated with the duration and severity of AKI and the duration of intensive care unit (ICU) stay after adult cardiac surgery, and have been identified as independent predictors of AKI, being superior to conventional biomarkers like s-Cr concentration (34). However, further studies involving these biomarkers have produced conflicting results (35) and these tests are expensive and not readily accessible. In contrast, s-Cr could be widely applicable due to it is cheap and readily available to most practitioners. Despite its relative non-specificity, s-Cr remains the gold standard for defining AKI.
Preoperative demographic risk factors that have been associated with the development of AKI after cardiac surgery include preexisting kidney disease (21,36-38), reduced left ventricular function (37-40), chronic obstructive pulmonary disease (COPD) (37,38), diabetes mellitus (37,39,41), older age (21,36,39-42), and women (37,41,43). Our study showed that women were more likely to develop AKI than men, which differs from the findings of Neugarten and colleagues’s (44) study but concurred with the results of Rosner’s study (45). Anatomically, women’s number of glomerulus is less than men’s. This fact may contribute to higher occurrence of AKI at women. Our results demonstrated that BSA and body weight were associated with the development of AKI similar to previously published papers. Kumar and colleagues (46) identified class III obesity [body mass index (BMI) >40 kg/m2] as an independent predictor of AKI after on-pump CABG. Obesity increases oxidative stress and endothelial dysfunction, and inflammation (47,48). Frederic T and colleagues (49) suggested increased oxidative stress may partially account for the risk for AKI associated with obesity. Thus, increased BMI predicts an increased risk for AKI after cardiac surgery. In addition, the administration of N-acetylcysteine to protect the kidney from oxidative stress is not recommended.
Other studies suggest that intraoperative factors such as cardiopulmonary bypass (CPB) times are important contributors to postoperative renal dysfunction (50-52). In our study, emergency operations caused higher risk of AKI than elective cases. This might be due in part to prolonged CPB, prolonged hypo-perfusion of renal medulla, and increased levels of vasoconstrictors. Patients who undergo emergency surgery often have altered hemodynamics, are more likely to receive potent drugs that may affect the s-Cr level, and are more likely to have a complicated postoperative course at ICU.
Some studies suggest that creatinine measured after coronary angiography might be useful for prediction of AKI and mortality after cardiac surgery (53). Another study found that AKI after coronary angiography was associated with a long-term decline in renal function and that the risk of progression to ESRD was 12-fold higher in patients with mild AKI than in patients without AKI (54).
The Coronary Artery Bypass Grafting Surgery Off- or On-pump Revascularization Study trial, which randomized 4,752 patients to on- versus off-pump CABG (OPCAB) and showed no difference in survival or any other meaningful clinical outcome, including new renal failure requiring RRT (55). Despite several large, retrospective studies, the answer is still unclear. Some of studies support a lower risk for AKI in patients who undergo OPCAB, especially patients with pre-existing renal dysfunction (56-59). On the other hand, renal function is not affected by the technique of CABG, whether with or without CPB, in spite of the theoretical advantage of off-pump surgery (60). Additionally, OPCAB was not associated with decreased rates or reduced severity of AKI in elderly patients (61).
Our results revealed that preoperative proteinuria is an independent predictor for AKI after isolated on-pump CABG. The considerable number of studies revealed that pre-proteinuria was regarded as a risk factor after cardiac surgery. Our study corresponds with the results of Huang and colleagues (62) demonstrating that preoperative proteinuria is an independent risk factor for the development of CSA-AKI. Documented proteinuria with dipstick results of more than 1+ have been reported to be associated with the risk of CSA-AKI (62). Proteinuria has been identified as a marker of renal damage, regardless of whether the etiology of the primary disease is DM, hypertension, or glomerulopathy. Recently, Rabelink and colleagues (63) proposed that degradation of endothelial glycocalyx served as the key mechanism causing albuminuria. Endothelial glycocalyx is a layer of polysaccharide gel, which plays a role as a barrier against albumin filtration. Recent reports from large epidemiologic studies have shown that patients with proteinuria have a higher risk of adverse outcomes than those without proteinuria at the same stage of CKD (64,65). A previous small study about CABG showed proteinuria is a factor to affect long-term cardiovascular death (66). Wu and colleagues (67) reported that proteinuria is a powerful independent risk factor of long-term all-cause mortality and ESRD after CABG.
AKI risk assessment through routine check of proteinuria prior to surgery might result in reductions in AKI incidence postoperatively. This is important because urine dipstick is an inexpensive, readily available test. And early detection of proteinuria could easily be incorporated into clinical practice. Therefore, screening for proteinuria is an effective strategy to identify individual patients who will develop AKI in the postoperative period. Dipstick urinalysis reliably can predict albuminuria with sensitivities and specificities of greater than 99% (68). False-positive and false-negative results are usual in dipstick urinalysis. Nonetheless, the urine dipstick test is still recommended as an initial reasonable way for the evaluation of renal function and is widely used as screening in primary health care services, due to its simplicity and low-cost.
Most renal dysfunction after cardiac surgery is a temporary and reversible event. In fact, patients with mild AKI are usually responsive to medical therapy and eventually show spontaneous recovery. In our study, AKI patients with stage 1 showed an increased level of s-Cr, but returned to normal level within a few days. A prompt intervention in the postoperative management, especially avoiding additional renal insults and optimizing volume status, can prevent a higher progression of perioperative AKI, and the occurrence of the worst outcomes, including in-hospital mortality. Thus, intensive renal preservation during the perioperative period appears to provide sufficient renal protection. Continuous renal replacement therapy (CRRT) offers steady fluid removal and their intensity can be easily titrated for prevention or rapid administration of treatment of volume overload.
Early diagnosis of AKI is important; firstly, it will help identify patients for nephrology referral and secondly, it will allow for timely interventions, which could improve outcomes. Several studies have actually shown that early nephrology referral for patients who develop AKI result in improved outcomes (22,69,70).
However, the limitation of our study is that our model was designed for isolated on-pump CABG and the data are derived from a single center. Nevertheless, our study group was a homogenous isolated on-pump CABG population with well-defined criteria and objective outcome. Our study needs to be tested prospectively at multiple centers to substantiate its broad applicability.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of the Yeungnam University Hospital with the approval no. 2018-12-003.
References
- Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation 2009;119:2444-53. [Crossref] [PubMed]
- Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol 2004;15:1597-605. [Crossref] [PubMed]
- Rydén L, Ahnve S, Bell M, et al. Acute kidney injury following coronary artery bypass grafting: early mortality and postoperative complications. Scand Cardiovasc J 2012;46:114-20. [Crossref] [PubMed]
- Rydén L, Ahnve S, Bell M, et al. Acute kidney injury after coronary artery bypass grafting and long-term risk of myocardial infarction and death. Int J Cardiol 2014;172:190-5. [Crossref] [PubMed]
- Brown JR, Kramer RS, Coca SG, et al. Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann Thorac Surg 2010;90:1142-8. [Crossref] [PubMed]
- Ishani A, Nelson D, Clothier B, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med 2011;171:226-33. [Crossref] [PubMed]
- Ivert T, Holzmann MJ, Sartipy U. Survival in patients with acute kidney injury requiring dialysis after coronary artery bypass grafting. Eur J Cardiothorac Surg 2014;45:312-7. [Crossref] [PubMed]
- Conlon PJ, Stafford-Smith M, White WD, et al. Acute renal failure following cardiac surgery. Nephrol Dial Transplant 1999;14:1158-62. [Crossref] [PubMed]
- Ryckwaert F, Boccara G, Frappier JM, et al. Incidence, risk factors, and prognosis of a moderate increase in plasma creatinine early after cardiac surgery. Crit Care Med 2002;30:1495-8. [Crossref] [PubMed]
- Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013;17:204. [Crossref] [PubMed]
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for service program. N Engl J Med 2009;360:1418-28. [Crossref] [PubMed]
- Brown JR, Parikh CR, Ross CS, et al. Northern New England Cardiovascular Disease Study Group. Impact of perioperative acute kidney injury as a severity index for thirty-day readmission after cardiac surgery. Ann Thorac Surg 2014;97:111-7. [Crossref] [PubMed]
- Gallagher S, Jones DA, Lovell MJ, et al. The impact of acute kidney injury on midterm outcomes after coronary artery bypass graft surgery: a matched propensity score analysis. J Thorac Cardiovasc Surg 2014;147:989-95. [Crossref] [PubMed]
- Dardashti A, Ederoth P, Algotsson L, et al. Incidence, dynamics, and prognostic value of acute kidney injury for death after cardiac surgery. J Thorac Cardiovasc Surg 2014;147:800-7. [Crossref] [PubMed]
- Olsson D, Sartipy U, Braunschweig F, et al. Acute kidney injury following coronary artery bypass surgery and long-term risk of heart failure. Circ Heart Fail 2013;6:83-90. [Crossref] [PubMed]
- Mariscalco G, Cottini M, Dominici C, et al. The effect of timing of cardiac catheterization on acute kidney injury after cardiac surgery is influenced by the type of operation. Int J Cardiol 2014;173:46-54. [Crossref] [PubMed]
- Mariscalco G, Nicolini F, Scannapieco A, et al. Acute kidney injury after composite valve-graft replacement for ascending aorta aneurysms. Heart Vessels 2013;28:229-36. [Crossref] [PubMed]
- Kuitunen A, Vento A, Suojaranta-Ylinen R, et al. Acute renal failure after cardiac surgery: Evaluation of the RIFLE classification. Ann Thorac Surg 2006;81:542-46. [Crossref] [PubMed]
- Kiers HD, van den Boogaard M, Schoenmakers MC, et al. Comparison and clinical suitability of eight prediction models for cardiac surgery-related acute kidney injury. Nephrol Dial Transplant 2013;28:345-51. [Crossref] [PubMed]
- Bastin AJ, Ostermann M, Slack AJ, et al. Acute kidney injury after cardiac surgery according to Risk/ Injury/Failure/Loss/End-stage, Acute Kidney Injury Network, and Kidney Disease: Improving Global Outcomes classifications. J Crit Care 2013;28:389-96. [Crossref] [PubMed]
- Haase M, Bellomo R, Matalanis G, et al. A comparison of the RIFLE and Acute Kidney Injury Network classifications for cardiac surgery-associated acute kidney injury: A prospective cohort study. J Thorac Cardiovasc Surg 2009;138:1370-6. [Crossref] [PubMed]
- Benedetto U, Luciani R, Goracci M, et al. Miniaturized cardiopulmonary bypass and acute kidney injury in coronary artery bypass graft surgery. Ann Thorac Surg 2009;88:529-5. [Crossref] [PubMed]
- Li SY, Chen JY, Yang WC, et al. Acute kidney injury network classification predicts in-hospital and long-term mortality in patients undergoing elective coronary artery bypass grafting surgery. Eur J Cardiothorac Surg 2011;39:323-8. [Crossref] [PubMed]
- Machado MN, Miranda RC, Takakura IT, et al. Acute kidney injury after on-pump coronary artery bypass graft surgery. Arq Bras Cardiol 2009;93:247-52. [PubMed]
- Chertow GM, Levy EM, Hammermeister KE, et al. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med 1998;104:343-8. [Crossref] [PubMed]
- Coca SG, Yalavarthy R, Concato J, et al. Biomarkers for the diagnosis and risk stratification of acute kidney injury: A systematic review. Kidney Int 2008;73:1008-16. [Crossref] [PubMed]
- Ponce D, Zorzenon Cde P, dos Santos NY, et al. Early nephrology consultation can have an impact on outcome of acute kidney injury patients. Nephrol Dial Transplant 2011;26:3202-6. [Crossref] [PubMed]
- Liotta M, Olsson D, Sartipy U, et al. Minimal changes in postoperative creatinine values and early and late mortality and cardiovascular events after coronary artery bypass grafting. Am J Cardiol 2014;113:70-5. [Crossref] [PubMed]
- Brown JR, Cochran RP, MacKenzie TA, et al. Long-term survival after cardiac surgery is predicted by estimated glomerular filtration rate. Ann Thorac Surg 2008;86:4-11. [Crossref] [PubMed]
- Kidney Disease. Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl 2012;2:1-138.
- Hoste EA, Kellum JA. Acute renal failure in the critically ill: impact on morbidity and mortality. Contrib Nephrol 2004;144:1-11. [Crossref] [PubMed]
- Tolpin DA, Collard CD, Lee VV, et al. Subclinical changes in serum creatinine and mortality after coronary artery bypass grafting. J Thorac Cardiovasc Surg 2012;143:682-8.e1. [Crossref] [PubMed]
- Ho J, Reslerova M, Gali B, et al. Serum creatinine measurement immediately after cardiac surgery and prediction of acute kidney injury. Am J Kidney Dis 2012;59:196-201. [Crossref] [PubMed]
- Haase M, Bellomo R, Devarajan P, et al. Novel biomarkers early predict the severity of acute kidney injury after cardiac surgery in adults. Ann Thorac Surg 2009;88:124-30. [Crossref] [PubMed]
- Vanmassenhove J, Vanholder R, Nagler E, et al. Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrol Dial Transplant 2013;28:254-73. [Crossref] [PubMed]
- Andersson LG, Ekroth R, Bratteby LE, et al. Acute renal failure after coronary surgery—a study of incidence and risk factors in 2009 consecutive patients. Thorac Cardiovasc Surg 1993;41:237-41. [Crossref] [PubMed]
- Thakar CV, Arrigain S, Worley S, et al. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol 2005;16:162-8. [Crossref] [PubMed]
- Chertow GM, Lazarus JM, Christiansen CL, et al. Preoperative renal risk stratification. Circulation 1997;95:878-84. [Crossref] [PubMed]
- Mangano CM, Diamondstone LS, Ramsay JG, et al. The Multicenter Study of Perioperative Ischemia Research Group. Renal dysfunction after myocardial revascularization: Risk factors, adverse outcomes, and hospital resource utilization. Ann Intern Med 1998;128:194-203. [Crossref] [PubMed]
- Ortega-Loubon C, Fernández-Molina M, Pañeda-Delgado L, et al. Predictors of postoperative acute kidney injury after coronary artery bypass graft surgery. Braz J Cardiovasc Surg 2018;33:323-9. [PubMed]
- Robert AM, Kramer RS, Dacey LJ, et al. Northern New England Cardiovascular Disease Study Group. Cardiac surgery-associated acute kidney injury: A comparison of two consensus criteria. Ann Thorac Surg 2010;90:1939-43. [Crossref] [PubMed]
- Zanardo G, Michielon P, Paccagnella A, et al. Acute renal failure in the patient undergoing cardiac operation. Prevalence, mortality rate, and main risk factors. J Thorac Cardiovasc Surg 1994;107:1489-95. [PubMed]
- Thakar CV, Liangos O, Yared JP, et al. Predicting acute renal failure after cardiac surgery: Validation and re-definition of a risk-stratification algorithm. Hemodial Int 2003;7:143-7. [Crossref] [PubMed]
- Neugarten J, Sandilya S, Singh B, et al. Sex and the Risk of AKI Following Cardio-thoracic Surgery: A Meta-Analysis Clin J Am Soc Nephrol 2016;11:2113-22. [Crossref] [PubMed]
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006;1:19-32. [Crossref] [PubMed]
- Kumar AB, Bridget Zimmerman M, Suneja M. Obesity and post-cardiopulmonary bypass-associated acute kidney injury: a single-center retrospective analysis. J Cardiothorac Vasc Anesth 2014;28:551-6. [Crossref] [PubMed]
- Silver AE, Beske SD, Christou DD, et al. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47(phox) expression and evidence of endothelial oxidative stress. Circulation 2007;115:627-37. [Crossref] [PubMed]
- Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 2006;83:461S-5S. [Crossref] [PubMed]
- Billings FT 4th, Pretorius M, Schildcrout JS, et al. Obesity and Oxidative Stress Predict AKI after Cardiac Surgery Am Soc Nephrol 2012;23:1221-8.
- Mangano CM, Diamondstone LS, Ramsay JG, et al. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med 1998;128:194-203. [Crossref] [PubMed]
- D’Onofrio A, Cruz D, Bolgan I, et al. RIFLE criteria for cardiac surgery-associated acute kidney injury: risk factors and outcomes. Congest Heart Fail 16 Suppl 2010;1:S32-6.
- Rodrigues AJ, Evora PR, Bassetto S, et al. Risk factors for acute renal failure after heart surgery. Rev Bras Cir Cardiovasc 2009;24:441-6. [Crossref] [PubMed]
- Garcia S, Ko B, Adabag S. Contrast- Induced Nephropathy and Risk of Acute Kidney Injury and Mortality After Cardiac Operations. Ann Thorac Surg 2012;94:772-6. [Crossref] [PubMed]
- James MT, Ghali WA, Tonelli M, et al. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int 2010;78:803-9. [Crossref] [PubMed]
- Lamy A, Devereaux PJ, Prabhakaran D, et al. CORONARY Investigators. Effects of off-pump and on-pump coronary-artery bypass grafting at 1 year. N Engl J Med 2013;368:1179-88. [Crossref] [PubMed]
- Stallwood MI, Grayson AD, Mills K, et al. Acute renal failure in coronary artery bypass surgery: Independent effect of cardiopulmonary bypass. Ann Thorac Surg 2004;77:968-72. [Crossref] [PubMed]
- Fransen E, Maessen J, Dentener M, et al. Systemic inflammation present in patients undergoing CABP without extracorporeal circulation. Chest 1998;113:1290-5. [Crossref] [PubMed]
- Dybdahl B, Wahba A, Haaverstad R, et al. On-pump versus off-pump coronary artery bypass grafting: More heat-shock protein 70 is released after on-pump surgery. Eur J Cardiothorac Surg 2004;25:985-92. [Crossref] [PubMed]
- Wright G. Hemolysis during cardiopulmonary bypass: Update. Perfusion 2001;16:345-51. [Crossref] [PubMed]
- Tuttle KR, Worrall NK, Dahlstrom LR, et al. Predictors of ARF after cardiac surgical procedures. Am J Kidney Dis 2003;41:76-83. [Crossref] [PubMed]
- Reents W, Hilker W, Börgermann J, et al. Acute kidney injury after on-pump or off-pump coronary artery bypass grafting in elderly patients. Ann Thorac Surg 2014;98:9-14; discussion 14-5. [Crossref] [PubMed]
- Huang TM, Wu VC, Young GH, et al. Preoperative proteinuria predicts adverse renal outcomes after coronary artery bypass grafting. J Am Soc Nephrol 2011;22:156-63. [Crossref] [PubMed]
- Rabelink TJ, de Zeeuw D. The glycocalyx-linking albuminuria with renal and cardiovascular disease. Nat Rev Nephrol 2015;11:667-76. [Crossref] [PubMed]
- Hallan SI, Ritz E, Lydersen S, et al. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol 2009;20:1069-77. [Crossref] [PubMed]
- Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010;303:423-9. [Crossref] [PubMed]
- Orii K, Hioki M, Iedokoro Y, et al. Prognostic factors affecting clinical outcomes after coronary artery bypass surgery: analysis of patients with chronic kidney disease after 5.9 years of follow-up. J Nippon Med Sch 2011;78:156-65. [Crossref] [PubMed]
- Wu VC, Huang TM, Wu PC, et al. Preoperative Proteinuria Is Associated with Long-Term Progression to Chronic Dialysis and Mortality after Coronary Artery Bypass Grafting Surgery. PLoS One 2012;7:e27687. [Crossref] [PubMed]
- Woolhandler S, Pels RJ, Bor DH, et al. Dipstick urinalysis screening of asymptomatic adults for urinary tract disorders. I. Hematuria and proteinuria. JAMA 1989;262:1214-9. [Crossref] [PubMed]
- Mehta RL, McDonald B, Gabbai F, et al. Nephrology consultation in acute renal failure: does timing matter? Am J Med 2002;113:456-61. [Crossref] [PubMed]
- Balasubramanian G, Al-Aly Z, Moiz A, et al. Early nephrologist involvement in hospital-acquired acute kidney injury: a pilot study. Am J Kidney Dis 2011;57:228-34. [Crossref] [PubMed]