
Aprepitant for gefitinib-induced refractory pruritus in Chinese malignancy population
Introduction
Targeted medications have been showed significantly to improve the survival of patients with various malignancies (1). It is an important method for anti-cancer therapy. Its dermatologic adverse event (AE) may affect the majority of treated patients (2). Common dermatological AE associated with these medications are hair loss, skin rash, pruritus, mucositis, xerosis or fissures and paronychia, and hand and foot syndrome (3,4). Rash and Pruritus is an unpleasant feel (5), seriously affect the quality of life, even lead to medication reduction or withdrawal, and tumor progression. There is higher EGFR mutation in non-small cell lung cancer (NSCLC) in China, and it is commonly treated with gefitinib. It is reported that the highest incidence of rash and pruritus associated with gefitinib (6).
Substance P is an important neuro-mediator of pruritus. The keratinocytes of patients with chronic pruritus have an increased number of neurokinin 1 receptors (7). Targeted Medications might induce secretion of stem cell factors and increase the number of dermal mast cells in skin rashes (8).
Aprepitant is the world's first new generation of an oral neurokinin 1 receptor antagonist that was approved in the United States in 2003, which is used in combination with other antiemetics to prevent chemotherapy-induced nausea and emesis (CINV), associated with initial and repeated treatment of highly emetogenic antitumor chemotherapy (HEC). Later it was approved to prevent post-operative nausea and vomiting (PONV) and nausea and vomiting associated with moderate emetic chemotherapy (MEC).
A few studies have found that pruritus caused by targeted medications have good response to aprepitant, an oral neurokinin 1 receptor antagonist that blocks mast-cell degranulation caused by neurokinin 1 receptor (9,10). It has been suggested that the effects of aprepitant are related to its preventing of mast-cell activation in the skin (11), however, the dosage and course of treatment is not uniform (12). Daniele reported aprepitant decreased severe pruritus induced by erlotinib (13), however there was none study reported the efficacy of aprepitant in treatment with pruritus induced by gefitinib which was widely used as the first treatment in NSCLC in China. This is our first report of aprepitant for refractory pruritus caused by targeted medication-gefitinib. Each patient provided written informed consent, and was previously treated with glucocorticoids without effect. We aim to evaluate the efficacy of aprepitant on pruritus, and explore the dosage and course of treatment with refractory rash and pruritus.
Case presentation
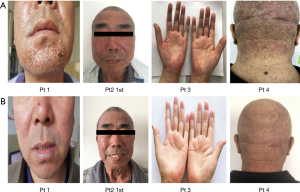
Between Jan 2017 and May 2017 in the 307th Hospital of Chinese PLA, we studied 4 NSCLC patients with severe skin rash and pruritus after resistance to standard treatment with steroids, antihistamines and Traditional Chinese Medicine. After inform consent, 4 patients took aprepitant (125 mg on day 1; 80 mg on day 2; 80 mg on day 3). Pruritus was assessed by VAS score in baseline, 7 days and 6 months after the first dose aprepitant. Skin photos were compared baseline to 7 days after the first dose aprepitant.
Table 1 shows characteristic of three males and one female, each of them with stage IV non-small cell lung cancer and receiving gefitinib at a dose of 250 mg daily, presented with rash and pruritus resistant to local application of glucocorticoids and to standard systemic therapies.

Full table
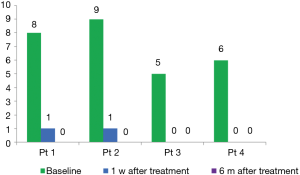
A 61-year-old man, patient no.2, with lung adenocarcinoma (cT4M3N1b, stage IV, exon 19 deletion) was received gefitinib. He has no smoking history and no medication allergies. The severe rash and pruritus seriously influenced his life quality within the first week after taking gefitinib. He firstly received local application of glucocorticoids, but there is no effect on it. Then we adjust the treatment with oral aprepitant, day 1, 125 mg; day 2, 80 mg; day 3 80 mg. He showed a prompt recovery from rash and pruritus in 24 hours after the first administration of aprepitant. Three additional patients received aprepitant for pruritus resistant to local application of glucocorticoids, and only one time administration was able to control the pruritus. We assessed the efficacy of aprepitant for pruritus via Visual Analogue Scale (VAS) score in 1 week and 6 months after first dose of aprepitant (seen in Figure 1). The pruritus was recovered and was compared in Figure 2.
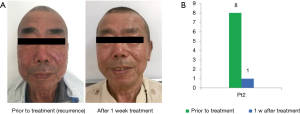
Patient2’s rash and pruritus recurred after two weeks, he took the next administration of aprepitant and rash and pruritus was completely recovered (seed in Figure 3). With 6 months follow-up visit from the first dose of aprepitant, there is no recurrence of pruritus.
Discussion
Pruritus is a common side effect of anti-EGFR antibodies and tyrosine kinase inhibitors used in cancer patients, primarily on the face, neck and upper torso. Studies showed that, including all levels of pruritus, the highest incidence of high-grade pruritus is gefitinib (6). Pruritus is an unpleasant skin AE, which seriously affecting the life quality of patients, chronic refractory pruritus also brings new challenges for cancer treatment, some patients with refractory pruritus has to stop targeted medication treatment, which increase the risk of tumor recurrence. Susan reported the results of a survey of 110 oncologists revealed that 76% of survey oncologists had held the EGFRI therapy owing to rash and pruritus, the drug was held up to 2–3 weeks (14).
As reported, EGFR tyrosine kinase inhibitors increase the number of dermal mast cells in skin rashes, which play an important role in pruritus. When activates the mast cells, the tryptase is released and to activated C fibres, which will transmit nerve impulse to central nervous system (15). There was crosstalk between C fibres and mast cell mediate by substance P, as known one kind of neuropeptides (16). Substance P participated in regulation of pruritus in two ways: first, mast cells will degranulation caused by high concentration of substance P bind to NK1 receptors expressed in the surface of mast cells (10); second, the binding also lead mast cells to product of tumor necrosis factors (TNF)-α, which is known to sensitive C-fibre terminal (17).
Aprepitant is a new generation of NK-1 antagonist, its anti-pruritus activity can be explained: first, Aprepitant can block the substance P to bind with NK-1 receptor in Mast cells in skin rash site.; second, substance P is a regulatory peptide found in areas of the central nervous system. Aprepitant is a potent and CNS-penetrant antagonist, maybe it can block the nerve impulse of pruritus in CNS.
The efficacy of aprepitant with treatment of pruritus has been confirmed. A few studies found the same conclusion: In 2009, Duval and Dubertret reported the first case of refractory pruritus treated with aprepitant of 80 mg, and the VAS score dropped from 8 to 2.33. All patients disappeared insomnia complain and had good sleep quality (18). Since then, treatment of aprepitant for refractory pruritus has been reported in the United States, South Korea, and Japan including cutaneous T-cell lymphoma, solid tumors, and many others indications. Many patients severely degrade their life quality due to pruritus-related side effects, insomnia, acne and so on. Most patients received routine treatment, but failed. However, almost all patients with aprepitant were relieved of pruritus symptoms including acne subsides, reduced insomnia, and VAS score dropped significantly (8,12,19).
Among these studies, there was limit data that aprepitant treated with pruritus induced by gefitinib in Asia population, especially in Chinese. Also there was no standard regimen of aprepitant in pruritus, the dosage and treatment cycle still to be investigated. Our study referenced to the standard treatment of aprepitant in CINV, we determined the regimen in this study: 125 mg on day 1; 80 mg on day 2; 80 mg on day 3, one cycle; the regimen will be repeated after pruritus relapse. We found that all the patients’ VAS scores decreased and skin acne was visually relieved (seen in Figure 2) after treatment, and this medication program has a good effect on pruritus without significant side effects. Based on our reports, aprepitant is a reliable option for the treatment of refractory pruritus due to gefitinib therapy to improve life quality of the patients.
There are some limitations in our study. The samples were limit in our study and to further validate the efficacy of aprepitant for refractory pruritus, large scale, randomized controlled trials are still needed. We only investigated the pruritus induced by gefitinib, and need to explore more type pruritus, such as induced by anti-CTLA 4 antibody, anti PD-1/PD-L1 antibody. One of our patients experienced recurred pruritus, so it’s important how to identify underlying pruritus in patients treated with EGFR inhibitors and select the patients with potential benefit be treated with aprepitant. These will be explored in our next study.
Conclusions
This is the first report of aprepitant for refractory pruritus caused by targeted medication in Chinese malignancy population. We confirmed the efficacy of aprepitant on refractory rash and pruritus, the dosage (125 mg on day 1; 80 mg on day 2; 80 mg on day 3) was well tolerated and easy to apply to supportive treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
References
- Ricciardi S, Tomao S, de Marinis F. Toxicity of targetedtherapy in non-small-cell lung cancer management. Clin Lung Cancer 2009;10:28-35. [Crossref] [PubMed]
- Balagula Y, Lacouture ME, Cotliar JA. Dermatologic toxicitiesof targeted anticancer therapies. J Support Oncol 2010;8:149-61. [PubMed]
- Lacouture ME, Anadkat MJ, Bensadoun RJ, et al. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer 2011;19:1079-95. [Crossref] [PubMed]
- Chan A, Tan EH. How well does the MESTT correlate with CTCAE scale for the grading of dermatological toxicities associated with oral tyrosine kinase inhibitors? Support Care Cancer 2011;19:1667-74. [Crossref] [PubMed]
- Liu T, Ji RR. New insights into the mechanisms of itch: are pain and itch controlled by distinct mechanisms? Pflugers Arch 2013;465:1671-85. [Crossref] [PubMed]
- Santoni M, Conti A, Andrikou K, et al. Risk of pruritus in cancer patients treated with biological therapies: A systematic review and meta-analysis of clinical trials. Crit Rev Oncol Hematol 2015;96:206-19. [Crossref] [PubMed]
- Yosipovitch PA. Pruritus. In: Lacouture ME, editor. Dermatologic principles and practice in oncology. Hoboken, NJ: John Wiley& Sons, Inc., 2014.
- Gerber PA, Buhren BA, Homey B. More on Aprepitant for Erlotinib-Induced Pruritus. N Engl J Med 2011;364:486-7. [Crossref] [PubMed]
- Ständer S, Siepmann D, Herrgott I, et al. The neurokinin receptor 1 with aprepitant: a novel antipruritic strategy. PLoS One 2010;5:e10968. [Crossref] [PubMed]
- Gerber PA, Buhren BA, Cevikbas F, et al. Preliminary evidence for a role of mast cells in epidermal growth factor receptor inhibitor-induced pruritus. J Am Acad Dermatol 2010;63:163-5. [Crossref] [PubMed]
- Wallengren J. Neuroanatomy and neurophysiology of itch. Dermatol Ther 2005;18:292-303. [Crossref] [PubMed]
- He A, Alhariri JM, Sweren RJ, et al. Aprepitant for the Treatment of Chronic Refractory Pruritus. Biomed Res Int 2017;2017:4790810. [Crossref] [PubMed]
- Santini D, Vincenzi B, Guida FM, et al. Aprepitant for management of severe pruritus related to biological cancer treatments: a pilot study. Lancet Oncol 2012;13:1020-4. [Crossref] [PubMed]
- Boone SL, Rademaker A, Liu D, et al. Impact and management of skin toxicity associated with anti-epidermal growth factor receptor therapy: survey results. Oncology 2007;72:152-9. [Crossref] [PubMed]
- Yosipovitch G, Greaves MV, Schmelz M. Itch. Lancet 2003;361:690-4. [Crossref] [PubMed]
- Suzuki R, Furuno T, McKay DM, et al. Direct neurite-mast cell communication in vitro occurs via the neuropeptide substance P. J Immunol 1999;163:2410-15. [PubMed]
- Cocchiara R, Lampiasi N, Albeggiani G, et al. Mast cell production of TNF-alpha induced by substance P evidence for a modulatory role of substance P-antagonists. J Neuroimmunol 1999;101:128-36. [Crossref] [PubMed]
- Duval A, Dubertret L. Aprepitant as an antipruritic agent? N Engl J Med 2009;361:1415-6. [Crossref] [PubMed]
- Chang SE, Han SS, Jung HJ, et al. Neuropeptides and their receptors in psoriatic skin in relation to pruritus. Br J Dermatol 2007;156:1272-7. [Crossref] [PubMed]


