
Tumor location is an independent prognostic factor of esophageal adenocarcinoma based on the eighth edition of TNM staging system in Chinese patients
Introduction
Esophageal cancer, mainly consisting of adenocarcinoma and squamous cell carcinoma (SCC), is the eighth most common malignant tumors and the sixth most common death cause of cancer worldwide (1). In the west, adenocarcinoma remains to be the predominant pathological type of esophageal cancer (2), while in China, adenocarcinoma was reported to account for only about 5–8% of all esophageal cancers (3,4). As a result, esophageal adenocarcinoma was rarely investigated among Chinese patients.
In the upcoming eighth edition of TNM staging system for esophageal cancer, tumor location of esophageal cancer was incorporated into the staging system only for esophageal SCC but not for adenocarcinoma (5). It is well established that tumor location is an independent prognostic factor of esophageal SCC (6). However, as for the prognostic value of tumor location for esophageal adenocarcinoma, previous studies have drawn controversial conclusions. Some reported that tumor location could significantly influence the survival of patients with esophageal adenocarcinoma (7,8), while others reported no impact of tumor location on the survival of esophageal adenocarcinoma patients (9-11). However, all those previous studies were based on previous TNM staging system (12), where the definition of tumor location differed significantly from the upcoming eighth edition of TNM staging system (5). For example, in the seventh edition, tumor location is defined by the position of the upper end of the cancer in esophagus (13), while in the eighth edition, it is defined by the cancer epicenter (5). More importantly, the definition of esophagogastric junction (EGJ) cancer also differed between the two editions. In the seventh edition, EGJ tumor contains Siewert types I, II, and III, while in the eighth edition, it only contains Siewert types I and II (14). Therefore, it is reasonable that previous studies have drawn controversial conclusions based on previous staging system. Since the eighth edition of TNM staging system has been available, it remains unknown whether tumor location has any impact on survival of esophageal adenocarcinoma patients based on the newest staging system (5). Moreover, due to the rarity of esophageal adenocarcinoma in Chinese patients, the prognostic value of tumor location in Chinese patients with esophageal adenocarcinoma has not been investigated yet. Therefore, in this study, we aimed to explore whether tumor location has any impact on the survival of Chinese patients with esophageal adenocarcinoma based on the eighth edition of TNM staging system. To our knowledge, our study is the first one applying the eighth edition of TNM staging system focusing on current topic and also the first study of Chinese patients with esophageal adenocarcinoma.
Methods
Data of patients with esophageal adenocarcinoma undergoing esophagectomy with lymphadenectomy in our department from January 2009 to December 2015 were retrospectively collected. The inclusion criteria are: (I) patients with pathologically and immunohistochemically diagnosed esophageal adenocarcinoma; (II) patients with resectable esophageal adenocarcinoma; (III) patients without distant metastasis. The exclusion criteria are: (I) patients with mixed pathologic types of esophageal cancer; (II) patients without complete data for analysis; (III) patients with Siewert type III esophageal adenocarcinoma (14). Our study was approved by the Ethics Committee of West China Hospital, Sichuan University (No. 20171230). Since our study is a retrospective prognosis analysis and analyzed anonymously, the ethics committee waived the need for consent.
The following data were collected for analysis: demographic data (including age and gender), comorbidity data (including hypertension, diabetes mellitus, mild to moderate narrow in coronary artery), pathologic findings, surgical approach, adjuvant therapy, and survival time. All those patients were restaged by the eighth edition of TNM staging system for esophageal adenocarcinoma (5). The pathologic findings consisted of pT stage, pN stage, lymphovascular invasion, tumor grade and tumor location. According to the eighth edition of TNM staging system (5), tumor location was categorized into four parts according to the endoscopic findings: cervical/upper (15 to 25 cm from the incisor teeth), middle (25 to 30 cm), and lower (30 to 40 cm) segments of the esophagus as well as EGJ. In order to explore the impact of tumor location on survival of these patients, we divided these patients into two groups according to the primary tumor site (adenocarcinomas at the EGJ (Siewert I/II) and adenocarcinomas at other sites of esophagus) due to the fact that most of the esophageal adenocarcinomas were located at the EGJ and that our study had a relatively limited sample size. All patients were followed up every 3 months for the first 2 years, then every 6 months for the following 3 years, and annually thereafter. Our last follow-up was conducted on telephone, through outpatient department visit, or home visit in December 2017. Survival time was measured from the day of esophagectomy to the date of death or last follow-up.
Data are presented as number and (%) for categorical data or mean ± standard deviation for continuous variables. For comparing continuously distributed data between groups, independent-sample Student’s t-test or the Mann-Whitney non-parametric U-test was applied; while for categorical data, Chi-squared test or Fisher’s exact test was applied. Survival time was calculated by the Kaplan-Meier analysis, and the log-rank test was used to explore the association between variables and survival time. The Cox’s hazard regression model was applied to investigate independent prognostic factors by entering variables with a P value of less than 0.2 in univariate analysis. In order to balance baseline characteristics between the two groups, propensity-score matched (PSM) analysis was performed using R-2.15.1-win and PSMATCHING 3.04 software. The propensity scores were calculated from a logistic regression model with covariates including age, gender, comorbidity, pTNM stage, tumor grade, surgical approach, and adjuvant therapy. Cases from the two groups were matched at a ratio of 1:1 by using the nearest-neighbor method with a caliper width of 0.2. All those statistical analyses were performed using IBM SPSS software (version 22.0; IBM Corp., Armonk, NY, USA). A two-sided P value less than 0.05 was considered statistically significant.
Results
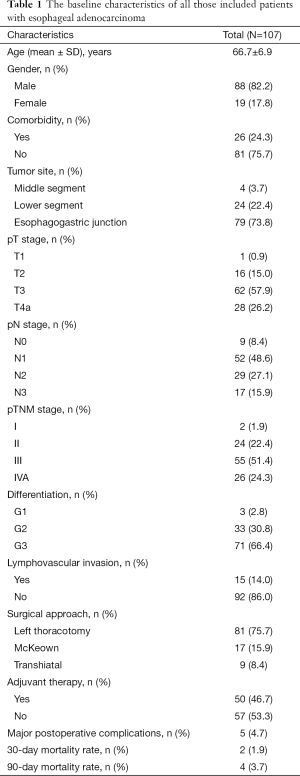
Baseline characteristics of all those patients
From January 2009 to December 2015, a total of 107 patients who consecutively underwent esophagectomy for esophageal adenocarcinoma and met our inclusion criteria were included for analysis. The baseline characteristics of all these patients are shown in Table 1. The mean age was 66.7±6.9 years old with a male to female ratio of 4.6:1. Twenty-six patients (24.3%) had comorbidity. The majority of the esophageal adenocarcinomas were located in the EGJ (73.8%) and lower (22.4%) segment of the esophagus. In pathology, most of those patients were in advanced pT stage (pT3/T4a: 84.1%) and were found to have positive lymph node metastasis (91.6%). Moreover, most of those patients were found to be poorly differentiated (66.4%) and 15 patients (14.0%) were found to have lymphovascular invasion. According to the eighth edition of TNM staging system, two patients were at stage I, 24 patients at stage II, 55 patients at stage III, and 26 patients at stage IVA. None of them received any neoadjuvant therapy because of their resectable esophageal disease, and all of them received radical esophagectomy with two-field lymphadenectomy. Because of the historical background of choosing left thoracotomy as a predominant approach for esophageal cancer in China, 81 (75.7%) patients underwent esophagectomy through left thoracotomy, seventeen patients via McKeown approach, and nine patients through transhiatal approach. Only 5 patients (4.7%) suffered from major postoperative complications (two for severe pneumonia, two for anastomosis leakage, and one for recurrent laryngeal nerve paralysis). Two patients (1.9%) died (one for severe pneumonia and another for esophagobronchial fistula) within 30 days after surgery. Moreover, the rate of 90-day mortality was 3.7%. Patients with advanced pT stage (pT3/T4a) or positive lymph node metastasis were all recommended for adjuvant therapy. However, only about half of those patients received adjuvant therapy postoperatively, while some patients who were recommended for adjuvant therapy declined it due to physical conditions or economic burdens.
Full table
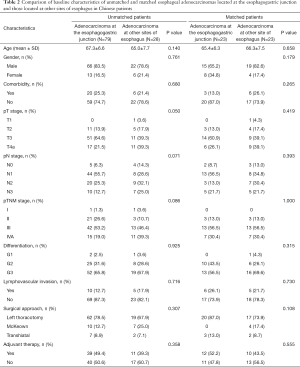
Comparison between unmatched cases of adenocarcinomas at the EGJ and these at other sites of esophagus
According to the location of primary tumor, we divided these patients simply into two groups (adenocarcinomas at the EGJ and adenocarcinomas at other sites of esophagus).The baseline characteristics of patients in the two groups are presented and compared in Table 2. Comparing to esophageal adenocarcinomas at the EGJ, adenocarcinomas at other sites of esophagus tended to present with more advanced pT stage (0.050) and pN stage (0.071). Moreover, there tended to be more tumors with stage IVA in esophageal adenocarcinomas at other sites of esophagus (39.3% vs. 19.0%, P=0.086). However, there were no significant differences of age (P=0.140), gender (P=0.761), rate of comorbidity (P=0.680), tumor differentiation (0.925), lymphovascular invasion (P=0.716), surgical approach (P=0.307), and adjuvant therapy application (P=0.358) between the two groups.
Full table
Survival analysis and prognosis
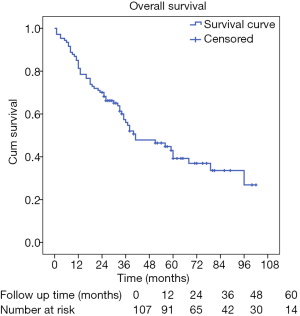
We conducted our last follow-up in December 2017 by telephone, outpatient department visit, or even patient visiting. The median follow-up time was about 60.0 months; 58 (54.2%) patients were decreased, while 41 (38.3%) patients were still alive, and 8 (7.5%) patients were lost to follow-up. The median survival time of these patients was 41.0 months (95% CI: 22.4–59.6 months), and the 1-, 3-, and 5-year survival rates for all these patients were 81.3%, 56.0%, and 39.2%, respectively (Figure 1).
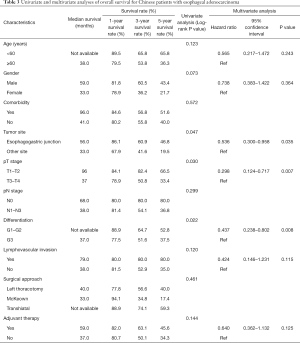
For prognosis, both univariate and multivariate analyses were applied (Table 3). Patients with esophageal adenocarcinoma in the EGJ had significantly longer survival time than these with esophageal adenocarcinoma in other sites of esophagus (5-year survival rate: 46.8% and 19.5%, respectively; Log-rank P=0.047; Figure 2). Patients with early pT stages (pT1/2) had significantly longer survival time than these with advanced pT stages (pT3/4a) (five-year survival rate: 66.5% and 33.4%, respectively; Log-rank P=0.030). Moreover, patients with poor tumor differentiation yielded significantly shorter survival time than these with moderate/well differentiated esophageal adenocarcinoma (5-year survival rate: 37.5% and 52.8%, respectively; Log-rank P=0.022). Patients of younger age (Log-rank P=0.123), male patients (Log-rank P=0.073), and patients receiving adjuvant therapy (Log-rank P=0.144) also tended to have longer survival time. However, no significant difference of survival time regarding to comorbidity (Log-rank P=0.572), pN stage (Log-rank P=0.299), lymphovascular invasion (Log-rank P=0.120), and surgical approach (Log-rank P=0.461) was observed.
Full table
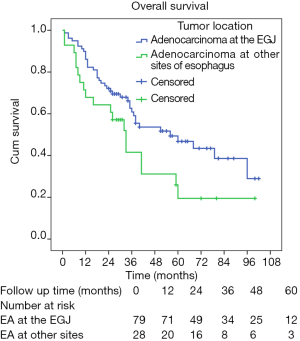
In the multivariate analysis, tumor site (P=0.035), pT stage (P=0.007), and tumor differentiation (P=0.008) were found to be independent prognostic factors for overall survival of these esophageal adenocarcinoma patients. Patients with esophageal adenocarcinomas in the EGJ [hazard ratio (HR) =0.536; 95% confidence interval (CI) =0.300–0.958; Figure 3], early pT stage (T1/T2) (HR =0.298; 95% CI =0.124–0.717), and moderate/well differentiated esophageal adenocarcinoma (HR =0.437; 95% CI =0.238–0.802) had significantly better overall survival. However, other factors including age (P=0.243), gender (P=0.364), lymphovascular invasion (P=0.115), and adjuvant therapy (P=0.125) were not found to be independent prognostic factors for these patients (Table 3).
Comparison between well-matched cases of patients with adenocarcinomas at the EGJ and these at other sites of esophagus
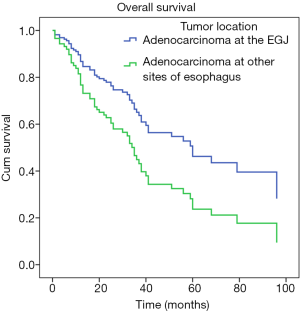
To investigate the actual impact of tumor location on survival of esophageal adenocarcinoma patients, PSM analysis was conducted by matching the following covariates: age, gender, comorbidity, pTNM stage, tumor grade, surgical approach, and adjuvant therapy. A total of 23 pairs were matched, and the baseline characteristics of the two groups are shown and compared in Table 2. All these baseline characteristics between adenocarcinomas at the EGJ and these at other sites of esophagus were comparable. For prognosis, patients with adenocarcinomas at the EGJ still had significantly longer survival time than these with adenocarcinomas at other sites of esophagus (5-year survival rate: 55.1% and 27.3%, respectively; Log-rank P=0.039; Figure 4).

Discussion
Esophageal adenocarcinoma has surpassed esophageal SCC becoming the predominant pathologic type of esophageal cancers in the West (2), while in China, adenocarcinoma was reported to make up only about 5–8% of all esophageal cancers (3,4). Therefore, the clinical characteristics and prognosis of esophageal adenocarcinoma in Chinese patients have been rarely reported (15). Hence, the prognostic value of tumor location in Chinese patients with esophageal adenocarcinoma has not been investigate yet. Although it has been widely explored in Western patients with esophageal adenocarcinoma, the prognostic value of tumor location remains controversial (9-11). More importantly, since the eighth edition of TNM staging system has just been introduced (5), it remains unknown whether tumor location has any impact on survival of esophageal adenocarcinoma patients based on the newest staging system. Therefore, in this study, we aim to figure out the clinical characteristics and prognosis of esophageal adenocarcinoma in Chinese patients and more importantly, to explore the impact of tumor location on the survival of these patients based on the eighth edition of TNM staging system. To our knowledge, this is the first study focusing on the prognostic value of tumor location by applying the eighth edition of TNM staging system focusing on current topic as well as the first study in Chinese patients with esophageal adenocarcinoma.
In our study, we included 107 patients with esophageal adenocarcinoma for analysis. The median follow-up time for all these patients was 60.0 months and the median survival time was 41.0 months. Both univariate and multivariate analyses confirmed that tumor location, pT stage, and tumor differentiation are independent prognostic factors of survival for these patients. However, the baseline characteristics of the two groups (adenocarcinomas at the EGJ and adenocarcinomas at other sites of esophagus) were not well balanced during comparison. Therefore, PSM analysis was applied to generate relatively well-matched pairs for analysis. Adjusted by PSM, tumor location was still found to have a significant impact on survival of esophageal adenocarcinoma patients. Therefore, our study found for the first time that tumor location could serve as an independent prognostic factor for esophageal adenocarcinoma in Chinese patients based on the eighth edition of TNM staging system.
Whether tumor location could serve as an independent prognostic factor of esophageal adenocarcinoma still remains controversial. Several studies have shown that tumor location did not significantly influence the survival of patients with esophageal adenocarcinoma (16-19). However, these studies (16-19) only compared adenocarcinomas in the distal esophagus (Siewert I) with these involving EGJ (Siewert II/III). Because all those esophageal adenocarcinomas belong to the EGJ tumors and are located closely to each other, it is reasonable that there was no significant survival difference among them. Previous studies (7-9,20-22) have also compared the survival of adenocarcinomas at the EGJ (Siewert I/II/III) with those at other sites of the esophagus. Some found that there was no significant difference of survival between the two groups (9,20,21), while others found that tumor location could significantly influence survival of esophageal adenocarcinoma patients (7,8,22). Fein et al. (22) even found that adenocarcinomas at the EGJ yielded significantly shorter survival than these at other sites of the esophagus. However, all these previous studies applied previous TNM staging system and significant differences of the definition of tumor location as well as EGJ tumor existed between previous staging system and the eighth staging system (5,13). Therefore, it is reasonable that previous studies have drawn controversial conclusions based on previous staging system. Since the eighth edition of TNM staging system has been available (5), it seems important to explore the prognostic value of tumor location based on the newest TNM staging system so as to provide up-to-date evidence. Therefore, we conducted our study based on the newest staging system and compared adenocarcinomas at the EGJ (Siewert I/II) with those at other sites of esophagus for the first time. We found that patients with adenocarcinomas at the EGJ had significantly better survival than these with adenocarcinomas at other sites of esophagus. Moreover, in all esophageal cancers, evidence has shown that survival increased with more distal location of the esophagus (23). Taken together, we believe that tumor location could serve as an independent prognostic factor for esophageal adenocarcinoma based on the eighth edition of TNM staging system, at least in Chinese patients. Possible explanation for the poor prognosis of patients with esophageal cancer at other sites of the esophagus may be explained as follow: first, adenocarcinomas at other sites of esophagus tended to infiltrate deeper than those at the EGJ, which indicated a more aggressive characteristics of adenocarcinomas at other sites of esophagus; second, similar to previous studies founding that tumor location was significantly associated with lymph node metastasis (24,25), our study also found that adenocarcinomas at other sites of esophagus tended to have more lymph node metastasis than those at the EGJ. Therefore, different surgical therapeutic strategies are indicated for esophageal adenocarcinomas with different tumor locations in the future. For example, neoadjuvant and adjuvant therapy might be more emphasized for esophageal adenocarcinomas located in other sites of esophagus. Moreover, similar to esophageal SCC, tumor location may need to be incorporated into TNM staging system for esophageal adenocarcinoma in the future.
In accordance with previous studies (9,26), we also found that pT stage and tumor differentiation had significant impact on the survival of esophageal adenocarcinoma patients. However, we did not find that pN stage and adjuvant therapy could significantly influence the survival of those patients. One possible reason is that in our study the majority (91.6%) of these patients had lymph node metastasis while only a small proportion of patients had no lymph node metastasis and this large unbalance of sample size could greatly influence overall results. Moreover, a large proportion of patients who were advised to have adjuvant therapy declined it. As a result, the causal relationship of adjuvant therapy application and survival has not been established in this study. Therefore, further similar studies are badly needed.
Limitations
Our study has several limitations. First, a relatively small sample size could decrease our analytical accuracy. Second, our retrospective study design could also impact the validity of overall results. Finally, we drew conclusions only based on Chinese patients, but whether similar results could be observed in Western patients needs further studies because the types of adenocarcinomas might be different between these two ethnic groups (15).
Conclusions
In this study, we investigated the prognostic value of tumor location for esophageal adenocarcinoma in Chinese patients based on the eight edition of TNM staging system for the first time. We found that esophageal adenocarcinomas in the EGJ yielded significantly longer survival than those in other sites of esophagus. Tumor location may serve as an independent prognostic factor of esophageal adenocarcinoma in Chinese patients based on the eighth edition of TNM staging system.
Acknowledgments
We thank sincerely for the efforts from Mr. Yiyang Zhang (Deyang College of Urban Rail Transit, Deyang, China) for the English edition of our study.
Funding: This work was supported by National Natural Science Foundation of China [No. 81672291; No. 31071210] (to YD Lin).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Our study was approved by the Ethics Committee of West China Hospital, Sichuan University (No. 20171230). Since our study is a retrospective prognosis analysis and analyzed anonymously, the ethics committee waived the need for consent.
References
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400-12. [Crossref] [PubMed]
- Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med 2014;371:2499-509. [Crossref] [PubMed]
- Zeng H, Zheng R, Zhang S, et al. Esophageal cancer statistics in China, 2011: Estimates based on 177 cancer registries. Thorac Cancer 2016;7:232-7. [Crossref] [PubMed]
- Liu S, Dai JY, Yao L, et al. Esophageal Adenocarcinoma and Its Rare Association with Barrett's Esophagus in Henan, China. PLoS One 2014;9:e110348. [Crossref] [PubMed]
- Rice TW, Ishwaran H, Ferguson MK, et al. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. J Thorac Oncol 2017;12:36-42.
- Shi H, Zhang K, Niu ZX, et al. Does tumour location influence postoperative long-term survival in patients with oesophageal squamous cell carcinoma? Eur J Cardiothorac Surg 2015;48:266-72. [Crossref] [PubMed]
- Eloubeidi MA, Desmond R, Arguedas MR, et al. Prognostic factors for the survival of patients with esophageal carcinoma in the U.S.: the importance of tumor length and lymph node status. Cancer 2002;95:1434-43. [Crossref] [PubMed]
- Agoston AT, Zheng Y, Bueno R, et al. Predictors of Disease Recurrence and Survival in Esophageal Adenocarcinomas With Complete Response to Neoadjuvant Therapy. Am J Surg Pathol 2015;39:1085-92. [Crossref] [PubMed]
- Yoon HH, Khan M, Shi Q, et al. The prognostic value of clinical and pathologic factors in esophageal adenocarcinoma: a mayo cohort of 796 patients with extended follow-up after surgical resection. Mayo Clin Proc 2010;85:1080-9. [Crossref] [PubMed]
- Shridhar R, Freilich J, Hoffe SE, et al. Single-institution retrospective comparison of preoperative versus definitive chemoradiotherapy for adenocarcinoma of the esophagus. Ann Surg Oncol 2014;21:3744-50. [Crossref] [PubMed]
- Sepesi B, Schmidt HE, Lada M, et al. Survival in Patients With Esophageal Adenocarcinoma Undergoing Trimodality Therapy Is Independent of Regional Lymph Node Location. Ann Thorac Surg 2016;101:1075-80; discussion 80-1. [Crossref] [PubMed]
- Yam PC, Tong D, Law S. Comparisons of sixth and seventh edition of the American Joint Cancer Committee staging systems for esophageal cancer. Ann Surg Oncol 2014;21:583-8.
- Li Z, Rice TW. Diagnosis and staging of cancer of the esophagus and esophagogastric junction. Surg Clin North Am 2012;92:1105-26. [Crossref] [PubMed]
- Siewert JR, Stein HJ. Carcinoma of the gastroesophageal junction - classification, pathology and extent of resection. Dis Esophagus 1996;9:173-82.
- Huang Q, Fan X, Agoston AT, et al. Comparison of gastro-oesophageal junction carcinomas in Chinese versus American patients. Histopathology 2011;59:188-97. [Crossref] [PubMed]
- Whitson BA, Groth SS, Li Z, et al. Survival of patients with distal esophageal and gastric cardia tumors: a population-based analysis of gastroesophageal junction carcinomas. J Thorac Cardiovasc Surg 2010;139:43-8. [Crossref] [PubMed]
- Onate-Ocana LF, Milan-Revollo G, Aiello-Crocifoglio V, et al. Treatment of the adenocarcinoma of the esophagogastric junction at a single institution in Mexico. Ann Surg Oncol 2007;14:1439-48. [Crossref] [PubMed]
- Leers JM, DeMeester SR, Chan N, et al. Clinical characteristics, biologic behavior, and survival after esophagectomy are similar for adenocarcinoma of the gastroesophageal junction and the distal esophagus. J Thorac Cardiovasc Surg 2009;138:594-602; discussion 601-2. [Crossref] [PubMed]
- Phillips AW, Lagarde SM, Navidi M, et al. Impact of Extent of Lymphadenectomy on Survival, Post Neoadjuvant Chemotherapy and Transthoracic Esophagectomy. Ann Surg 2017;265:750-6. [Crossref] [PubMed]
- Talsma AK, Ong CA, Liu X, et al. Location of lymph node involvement in patients with esophageal adenocarcinoma predicts survival. World J Surg 2014;38:106-13. [Crossref] [PubMed]
- Messager M, Neofytou K, Chaudry MA, et al. Prognostic impact of preoperative platelets to lymphocytes ratio (PLR) on survival for oesophageal and junctional carcinoma treated with neoadjuvant chemotherapy: A retrospective monocentric study on 153 patients. Eur J Surg Oncol 2015;41:1316-23. [Crossref] [PubMed]
- Fein R, Kelsen DP, Geller N, et al. Adenocarcinoma of the esophagus and gastroesophageal junction. Prognostic factors and results of therapy. Cancer 1985;56:2512-8. [Crossref] [PubMed]
- Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus 2009;22:1-8. [Crossref] [PubMed]
- Mine S, Sano T, Hiki N, et al. Thoracic lymph node involvement in adenocarcinoma of the esophagogastric junction and lower esophageal squamous cell carcinoma relative to the location of the proximal end of the tumor. Ann Surg Oncol 2014;21:1596-601. [Crossref] [PubMed]
- Yamada M, Oda I, Tanaka H, et al. Tumor location is a risk factor for lymph node metastasis in superficial Barrett's adenocarcinoma. Endosc Int Open 2017;5:E868-e74. [Crossref] [PubMed]
- Rice TW, Chen LQ, Hofstetter WL, et al. Worldwide Esophageal Cancer Collaboration. pathologic staging data. Dis Esophagus 2016;29:724-33. [Crossref] [PubMed]


