
Clinical features of Wilson disease
A pathogenetic view and natural history
The clinical presentation of Wilson disease (WD) depends on the natural history of the disorder which reflects the pathogenesis. However, this is not an entirely elucidated mechanism. Therefore, we present here only one likely hypothesis.
Due to the defect/lack of ATP7B as the copper transporter from cytosol to the trans-Golgi-network (TGN), copper accumulates within cytoplasm where it is bound to metallothionein (MT). Although MT synthesis is induced by copper, space is limited. Consequence is the deposition within lysosomes as copper aggregates, which is histologically detectable by Rhodamine staining. From now on, it takes several years before the storage capacity of the lysosomal compartment reaches its limits. Then they burst and release their acidic, copper loaded content to the cytosol, where it induces—possibly via free radical formation—cellular damage and even cell death. A fulminant hepatic failure can result, which was shown to be due to an apoptotic chain reaction (1). Copper, released from destructed hepatocytes, binds in serum loosely to albumin from where it is provided to other organs and tissues causing cell damage. In case of a rapid release of copper to serum, e.g., by fulminant hepatic failure, it might cause hemolytic anemia.
Initial clinical presentation and acute hepatic failure
There is indeed an estimated proportion of 30% of WD patients who are asymptomatic and are detected mostly by family screening. It is assumed that they remain in the pre-destructive phase of copper accumulation (Figure 1). However, the majority of the patients present with clinical symptoms. About half of these patients have hepatologic as well as neurologic manifestations. The remaining half presents either with simple liver disease or pure neuronal disorders with a latent liver manifestation. Epidemiologic analyses show that in children, adolescents and young adults, WD is the most common reason for hemolytic anemia and cirrhosis, despite its rare frequency in the entire population of 1:30,000 (2).
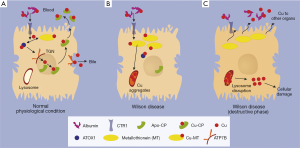
It is indeed our experience that fulminant hepatic failure as initial presentation is a frequent event in the young population of WD. Generally affected are more female than male patients although in the overall WD patient population there is an equal gender distribution (3). Hemolytic anemia and fulminant hepatic failure are due to the proposed burst of lysosomes overloaded with copper which is accompanied by release of unbound (free) copper into blood. There it is only loosely bound to albumin (non-ceruloplasmin-bound copper) and can attack erythrocyte plasma membranes resulting in a Coombs-negative hemolysis or finally a hemolytic crisis. Under these circumstances, the urinary copper excretion of the “free” copper is high. However, it has to be considered that acute liver failure is often associated with elevated urinary copper or alternatively with kidney failure and anuria (4). Low ceruloplasmin is a diagnostic hallmark of WD. However, ceruloplasmin may be in the normal range in hepatic failure because it reacts as an acute phase reactant. Therefore, this value is not reliable for diagnosis of WD in an acute setting. Moreover, coagulation is severely impaired in liver failure, particularly in WD. Thus, liver biopsies for quantitative determination of the liver copper content are risky, if coagulation factors and platelets are not adequately substituted. Genetic testing as another diagnostic option is not always conclusive and usually the procedure is time lasting within the short period of time before death may occur. Therefore, diagnosis of fulminant WD may be a challenge. It is the clinical picture which suggests the diagnosis. Typical is the presentation of a young female patient with deep jaundice and an unremarkable history. The bilirubin is high (about 50% conjugated/50% unconjugated), whereas alkaline phosphatase is surprisingly low in some, but not all cases (4). Transaminases are only marginally elevated (AST > ALT). Despite severely impaired coagulation as sign of organ failure, hepatic encephalopathy is only seen in very late stages of hepatic decompensation. Hemolytic anemia is another frequent finding (Table 1). For fulminant hepatic failure a high-urgency liver transplantation is required. Fortunately, the outcome of liver transplantation is very favorable, copper metabolism normalizes quickly after transplant and most of respective patients show excellent post liver transplantation survival both at one year and long-term. The explanted liver reveals—despite the clinical appearance as acute liver failure—in most cases a cirrhotic architecture. The elevated liver copper content confirms finally the diagnosis.

Full table
Clinical presentation beyond acute hepatic failure
In most cases (>90% of patients) the redistribution of hepatic lysosomal copper to cytoplasm is a slow process. It primarily hits the liver itself through free radical injury causing cell damage, consequent development of fibrosis or even cirrhosis. Hepatocellular carcinoma in the rather young patient population is a rare event. Patients with WD almost always have a prominent splenomegaly with concomitant thrombocytopenia. It is in part due to portal hypertension and in part due to hemolysis.
According to the proposed pathogenesis (Figure 1), there is a constant spillover of free copper to serum (albumin bound copper). This is taken up by other organs via the plasma membrane localized copper transporter CTR1, causing the clinical manifestation of WD (Table 2). Most affected is the brain with consequent copper deposition in the basal ganglia resulting in motoric disturbances (hepatolenticular degeneration) (Table 3) (5). The fine motoric skills and coordination are often affected resulting in micrography, deterioration of writing skills, tremor, dysphagia, ataxia, nystagmus and frequently speech disturbance. Later also gross motor skills are in disorder with choreoathetoidic dyskinesia, dystonia, uncontrolled movements, spastic contractions, gait abnormalities and finally disability. In late stages, features are retraction of upper lip and hypersalivation.
Full table

Full table
Approximately 30–60% of WD patients show psychiatric symptoms at presentation (6). Psychiatric manifestations may include depressive mood, cognitive and affective disorders. Rarely also psychotic episodes can occur (7). Of note, WD can be present over years with psychiatric symptoms without any clinical signs of hepatic or neurologic disease (6).
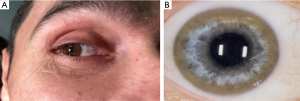
The deposition in the Descemet’s membrane of the cornea results in Kayser-Fleischer corneal rings (Figure 2) or infrequently in sun flower cataracts. Kayser-Fleischer (KF) rings do not impair the vision. Those deposits are seen in about 95% of patients with neurologic symptoms and are considered as pathognomonic for WD. In patients with primarily hepatic manifestation KF rings are detectable in only 50% (8). Similar, but less impressive rings are observed in rare cases in cholestatic liver diseases with moderate increase of copper in the system due to impaired biliary excretion (9).
The kidneys develop tubular injury and dysfunction. Renal tubular acidosis or an aminoaciduria, proteinuria, hyperuricosuria, hypercalciuria, hyperphosphaturia, uricosuria and glucosuria were reported. Occasionally, copper is also accumulated in cardiomyocytes and causes cardiomyopathy associated with arrhythmias. Osteoporosis and arthralgia are often registered in WD but are also very common in the general population. Rarely visible are lunulae and gigantism, the reason behind these remains unclear.
Conclusions
Clinical features of WD mainly relate to liver and brain. The development of cirrhosis is common but clinically apparent only in late stages. Due to the available therapies, liver disease is not as threatening as it has been until the 1950’s where it often ended with a fatal prognosis. Motoric neuropathy is a bigger problem because it is not always responsive to the decoppering therapies. The remaining clinical features span many disorders resulting from copper overload and cell damage of different tissues. These other manifestations are rare, like renal tubulopathy, cardiomyopathy or unspecific like osteoporosis or harmless like Kayser-Fleischer rings. Hemolysis and fulminant hepatic failure are important complications often observed in young patients as initial symptoms. To consider WD in these cases can direct the patients to the life-saving therapy of liver transplantation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Strand S, Hofmann WJ, Grambihler A, et al. Hepatic failure and liver cell damage in acute Wilson's disease involve CD95 (APO-1/Fas) mediated apoptosis. Nat Med 1998;4:588-93. [Crossref] [PubMed]
- European Association for Study of Liver. EASL Clinical Practice Guidelines: Wilson's disease. J Hepatol 2012;56:671-85. [Crossref] [PubMed]
- Ferenci P, Stremmel W, Czlonkowska A, et al. Age, sex, but not ATP7B genotype effectively influences the clinical phenotype of Wilson disease. Hepatology 2018. [Epub ahead of print].
- Eisenbach C, Sieg O, Stremmel W, et al. Diagnostic criteria for acute liver failure due to Wilson disease. World J Gastroenterol 2007;13:1711-4. [Crossref] [PubMed]
- Morbus Wilson HW. Extrapyramidalmotorische Störungen. In: Diener HC, Weimar C. editors. Leitlinien für Diagnostik und Therapie in der Neurologie. Thieme Verlag, 2012.
- Zimbrean PC, Schilsky ML. Psychiatric aspects of Wilson disease: a review. Gen Hosp Psychiatry 2014;36:53-62. [Crossref] [PubMed]
- Zimbrean P, Seniow J. Cognitive and psychiatric symptoms in Wilson disease. Handb Clin Neurol 2017;142:121-40. [Crossref] [PubMed]
- Pandey N, John S. Kayser-Fleischer Ring. StatPearls. Treasure Island, USA: 2018.
- Czlonkowska A, Litwin T, Dusek P, et al. Wilson disease. Nat Rev Dis Primers 2018;4:21. [Crossref] [PubMed]


