
Significant association of CD4+CD25+Foxp3+ regulatory T cells with clinical findings in patients with systemic lupus erythematosus
Introduction
The T lymphocyte subset with negative regulatory activity plays an important role in the mechanism which maintains self-tolerance and immune homeostasis. CD4+CD25+Foxp3+ regulatory T cells (Treg) are the best type characterized for this functional population (1,2). It has been proven that defects in these CD4+Treg cells in the number or/and function are involved in the pathogenesis of autoimmune diseases such as rheumatoid arthritis and multiple sclerosis (3-6). Systemic lupus erythematosus (SLE) is an important example of an autoimmune disease and is characterized by a loss of immune tolerance to self-antigens, including over-activation of auto-reactive T and B cells, auto-antibody production, and immune inflammation in multiple tissues. Thus far, a great deal of research has focused on the role of Treg in the pathogenesis of SLE, but the data from the series reported has been controversial (7). Some earlier studies reported a decrease in the amount of CD4+Treg in the peripheral blood of SLE patients (8,9). Another study showed the level of CD4+ CD25brightTreg in SLE patients was similar to that found in healthy controls, although some patients showed the defective regulatory function of the CD4+CD25+T subset (10). Moreover, some researchers found Treg cells with normal function in SLE patients (9,11). Later, more researchers demonstrated that there existed a reduced number and impaired function of CD4+Treg obtained in SLE patients (12,13). However, it seems paradoxical that an expansion of CD4+Treg was found in SLE (14,15). Thus, a definitive conclusion has not been drawn (15,16). Here, we investigated the changes of CD4+CD25+ Foxp3+Treg in the number and functional cytokine expressions by the CD4+CD25+ T subset in patients with SLE and analyzed their correlations with disease activity and clinical features. Our data showed a definite decrease of CD4+CD25+Foxp3+Treg number, and this decrease was correlated with the SLE Disease Activity Index (SLEDAI), immunological abnormalities and types of tissue damage.
Methods
Patients and controls
Forty-five patients (41 women and 4 men; mean age 33.8±9.8 years) who fulfilled the American College of Rheumatology (ACR) criteria for the classification of systemic lupus erythematosus (17) were enrolled in the study after giving informed consent. The patients with SLE were recruited from the authors’ affiliated institution and were divided into 3 groups based on their SLEDAI score (18). The first group had 25 patients with high-activity (SLEDAI >9; 23 females and 2 males) and with a mean age of (36.2±9.4) years. The second group was composed of 10 patients with low-activity (5≤ SLEDAI ≤9; 8 females and 2 males) and with a mean age of 38.4 (±11.6) years. The third group included 10 female patients with inactive SLE (SLEDAI <5) and with a mean age of 39.8 (±11.2) years. After giving informed consent, 40 healthy blood donors (31 women and 9 men) with a mean age of 30.2 (±5.6) years who had no history of autoimmune disease, were enrolled in our study as controls.
Cell and cell culture
Peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood (5 mL/patient) which was anti-agglutinated with heparin by Ficoll-histopaque 1077 (Sigma Chemical Co., Dorset, England). PBMCs were maintained in RPMI 1640 medium (Invitrogen Corp., Grand Island, NY, USA) containing 10% fetal bovine serum (Gibco, Carlsbad, CA, USA) with 5% CO2 at 37 °C. Then, some samples of PBMCs were used for testing CD4+CD25+Foxp3+ Treg, while others were used for CD3+ T-cell isolation through using human CD3+T cell negative sorting mini-magnetic beads (Dynal, USA) and a magnetic cell-sorting column (Miltenyi, Germany). The samples of CD3+ T cells were exposed to monensin (eBioscience, USA, 2 µM) with or without 20 µL/mL of ionomycin (Sigma, USA) together with PMA (Sigma, USA,25 ng/mL) for 5 hours for testing intracellular cytokines.
Flow cytometry
The fresh-isolated PBMCs were stained by Human Regulatory T Cell Staining Kit (eBioscience, USA) for Treg cells determination. They were briefly stained with FITC-anti-CD4 and APC-anti-CD25 antibodies, or their corresponding isotype control antibodies, and, after incubation with fixation/permeabilization working solution for 60 minutes, stained again with PE-anti-Foxp3 antibody or its corresponding isotype control antibody. In order to determine intracellular cytokines, the cultured CD3+ T-cells were stained with FITC-anti-CD4 and PE-anti-CD25, or the corresponding isotype control antibodies, for 30 minutes. Then, after washing twice, the cells were co-incubated with fixation/permeabilization working solution for 60 minutes. Also, 2 µL 2% of normal rat serum was added into the cells for a 15-minute blockade. Next, the cells were each stained with APC-conjugated anti-IFN-γ, anti-TGF-β or anti-IL-10, or the corresponding isotype control antibody (eBioscience, USA), and incubated for 30 minutes. After washing twice, the cells were resuspended in PBS and analyzed by flow cytometer (BD Biosciences). Finally, the data were read with FlowJo software (Tree Star, San Carlos, CA, USA).
Statistical analysis
All statistical analysis was performed using GraphPad Prism 6. Data are expressed as the mean ± standard deviation (SD). The unpaired t-test was used for two group comparisons, and ANOVA was used for analysis of differences in three or more groups. Correlation between two variables was evaluated using a linear correlation analysis. The statistical significance of correlation coefficients was calculated using the Spearman’s rho test. For all tests, P values less than 0.05 were considered significant.
Results
Reduction of CD4+CD25+Foxp3+Treg in PBMCs of SLE patients and its correlation with SLEDAI and clinical features
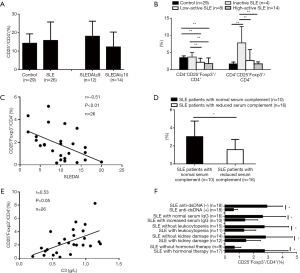
At first, we examined the CD4+CD25+Foxp3+T subset in fresh-isolated PBMCs from 26 of the patients with SLE and 29 healthy controls. The percentages of the CD4+CD25+ subset in the CD4+T cells in SLE patients did not show any significant differences as compared with healthy controls. Additionally, the percentages of the CD4+CD25+ subset in the CD4+T cells of high-active SLE patients were comparable to that of inactive and low-active SLE patients (Figure 1A). However, the percentages of the CD25+Foxp3+subset in the CD4+T cells in active SLE patients, both in low-active and high-active patients, decreased significantly as compared with inactive SLE patients and healthy controls (Figure 1B) and was inversely correlated with SLEDAI (Figure 1C). Moreover, the percentages of the CD25+ Foxp3+subset in the CD4+T cells of SLE patients were significantly correlated with a reduced level of serum complement (Figure 1D and 1E). Lower percentages of CD25+ Foxp3+subset in the CD4+T cells were observed in SLE patients with a positive anti-dsDNA antibody, increased serum level of IgG, leukocytopenia, kidney damage and without hormonal therapy when compared with their corresponding controls (Figure 1F). In contrast, the percentages of the CD25– Foxp3+subset in the CD4+T cells in inactive SLE patients were higher than those in active SLE patients and healthy controls (Figure 1B).
Abnormalities of functional cytokine expressions by CD4+CD25+T subset of SLE patients
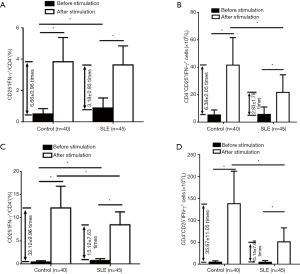
The function of Treg cells depends on cytokine production as well as Foxp3 expression. Because of the lower quantity of Foxp3+ cells in CD4+T cells, we tested intracellular cytokines, such as IFN-γ, TGF-β, and IL-10, in the CD4+CD25+ T subset, to compare with the CD4+CD25– T subset, using the isolated CD3+T-cell samples from the all 45 patients and 40 controls. First, we analyzed IFN-γ expression. Compared with healthy controls, SLE patients had slightly increased percentages of CD25+IFN-γ+ cells and CD25-IFN-γ+ cells in CD4+T cells fresh-isolated and without stimulation. After stimulation with PMA, ionomycin, and monensin for 5 hours, IFN-γ expression significantly increased, more obviously in the CD4+CD25-T subset than in the CD4+CD25+ T subset, representing a response to the stimulation (Figure 2). Furthermore, the percentages and the numbers of CD25+IFN-γ+ and CD25-IFN-γ+ cells, in CD4+ T cells were increased more significantly in healthy controls than those in SLE patients, indicating an impaired ability to produce IFN-γ by the CD4+CD25+ and CD4+CD25– T subsets upon stimulation in SLE patients (Figure 2). However, for SLE patients, neither the increased percentage of the CD4+CD25+ IFN-γ+ T subset, along with the CD4+CD25–IFN-γ+ T subset, fresh-isolated, nor the decrease of IFN-γ-producing response of CD4+CD25+ T subset, along with the CD4+CD25-IFN-γ+ T subset, to stimulation, were correlated with SLEDAI (data not shown).
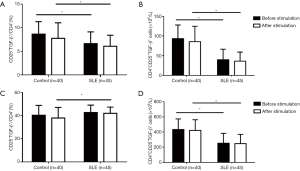
We then evaluated TGF-β expression by comparing the CD4+CD25+ T with the CD4+CD25-T subset. The results showed that in comparison to the CD4+CD25+ T subset, the CD4+CD25– T subset produced a higher level of TGF-β, regardless of the metric considered (percentage or number of TGF-β+ cells); the stimulation did not increase TGF-β expression, both by the CD4+CD25+ and CD4+CD25– T subsets, or both in healthy controls and SLE patients, indicating a relatively stable expression of TGF-β by these CD4+ T cell subsets (Figure 3). When compared with healthy controls, the percentages and the numbers of TGF-β+ cells in the CD4+CD25+T subset of SLE patients were significantly lower, and the numbers of CD4+CD25–TGF-β+ cells were also obviously lower, although the percentages of CD25–TGF-β+ cells in CD4+ T cells were slightly higher only after stimulation (Figure 3). Again, for SLE patients, the reduced TGF-β expression by the CD4+CD25+ T and CD4+CD25– T subset was not correlated with SLEDAI (data not shown).
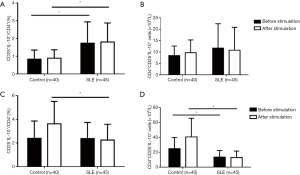
We also analyzed the productions of IL-10. We found that there were more cells expressing IL-10 in the CD4+CD25– T subset than in the CD4+CD25+ T subset, especially in response to the stimulation (Figure 4). Also, the percentages and numbers of CD4+CD25+IL-10+ cells did not significantly change both in SLE patients and healthy controls (Figure 4), indicating a stable expression of IL-10 by the CD4+CD25+T subset. In healthy controls, CD4+CD25–IL-10+ cells were clearly increased after stimulation, indicating a response of induced IL-10 expression by the CD4+CD25– T subset to the stimulation. The percentages of CD25+IL-10+ T subset in CD4+ T cells were found to be slightly increased, while the induced IL-10 expression by CD4+CD25– T subset in response to stimulation significantly decreased in SLE patients, regardless of whether considering the percentages or the numbers of CD4+CD25–IL-10+ cells (Figure 4). Importantly, for SLE patients, neither the slightly increased percentages of the CD4+CD25+IL-10+T subset nor the decreased percentages and the numbers of induced IL-10-expressing cells in the CD4+CD25-T subset by stimulation, were correlated with SLEDAI (data not shown).
Discussion
The role of CD4+Treg in SLE has not been satisfactorily elucidated. CD4+Treg cells have different subsets with different bio-markers and diverse functions. The bio-markers selected for examination will influence the results. As CD4 and CD25 have been considered to be the marker molecules for the identification of Treg, the conclusion drawn from these results would be partially incorrect; CD4+CD25+T cells are not really Tregs. T cells are capable of expressing CD25 when they are activated, which is the reason for the increased number of CD4+CD25+T cells in active SLE patients (19). Even CD25bright, which has been considered as a marker for identification of CD4+Treg, may lead to confusing results in the cases of the tumor and autoimmune inflammatory diseases such as SLE because CD4+Tregs defined by CD25bright cannot be differentiated from activated effector T cells (20,21). Foxp3 has been proven to be a characteristic molecule related to the development and function of Treg (22,23). Foxp3 is also thought of as a relatively specific marker used in defining CD4+Treg (6,11,24,25). In the present study, we chose both CD25 and Foxp3 as the markers of CD4+Treg. The result showed that the percentage of CD4+CD25+Foxp3+Treg decreased significantly in active SLE patients as compared with inactive SLE patients and healthy controls. The number of CD4+CD25+Foxp3+Treg was inversely correlated with SLEDAI and positively correlated with complement C3 level. The decreased number of CD4+CD25+Foxp3+ Treg were associated with a positive anti-dsDNA antibody, increased IgG level, leukocytopenia and kidney damage in SLE patients. These results clearly and definitely suggest that the deficiency of CD4+CD25+Foxp3+Treg in SLE patients was involved in the pathogenesis of SLE.
In the present study, we also found another interesting subset, CD4+CD25–Foxp3+, which displayed a higher percentage in inactive SLE patients when compared with active SLE patients and healthy controls. This is coincident with the report by Nocentini et al. (26). What are these CD4+ CD25–Foxp3+ cells? Yang and Zhang et al. reported an increased percentage of CD4+CD25–Foxp3+ cells in a group of untreated new-onset patients with SLE. Although these cells were CD127low, being coincident with Treg, they were considered to be non-Treg owing to the fact that these cells were positively correlated with the titer of anti-dsDNA antibody and decreased in most patients with active lupus after effective treatment (27,28). Meanwhile, Bonelli et al. described an increased CD4+CD25–Foxp3+ T subset with regulatory activity in SLE; these cells were also CD127low and suppressed T cell proliferation, and were suggested to be dysfunctional Treg (29). As to the contradiction between Yang’s and Zhang’s studies and Bonelli’s study, Horwitz suggested that it was due to the difference in studied patients, untreated new-onset patients and long-standing patients (30). Miyara divided CD4+CD25–Foxp3+ cells into three distinct populations based on CD45RA and CD25 expressions: CD45RA–CD4+CD25high Foxp3high, which is active or effector Treg (eTreg) with suppressive activity; and their precursor cells CD45RA+CD4+ CD25moderateFoxp3low, which is resting or naïve Treg without suppressive activity; and CD45RA-CD4+CD25dimFoxp3low, which may be conventional T cell, rather than Treg (25,31). Recently, Nocentini et al. demonstrated that there was a novel type of CD4+CD25low/- GITR+ regulatory T cell expanding particularly in inactive SLE patients, which expressed a high level of Foxp3 and exerted an inhibitory activity against autologous and heterologous effector T cells by a cell-contact-independent along with an IL-10 and TGF-β-mediated manner. These CD4+CD25low/- GITR+ regulatory T cells were suggested to be a homeostatic attempt to control activated effector T cells and played a key role in the modulation of the abnormal immune response in SLE (26). Therefore, the evaluation of Treg should be performed in combination not only with other markers and function assays but also with SLE disease activity and clinical features. A recent study by Huynh et al. demonstrated the control of PI3K (phosphatidylinositol-3-kinase) by PTEN influenced the homeostasis and lineage stability of Tregs (32). PTEN, the main negative regulator of PI3K, plays an important role in the control of PI3K activity. They found that control of PI3K in Treg cells was essential for lineage homeostasis and stability, and that diminished control of PI3K by PTEN in Treg cells led to reduced expression of IL-2 receptor α subunit CD25, enhanced accumulation of Foxp3+CD25– cells and, ultimately, loss of expression of Foxp3 in these cells. Therefore, we thought the increased CD25−Foxp3+ Treg subset in inactive SLE patients might represent a transitional population between activated CD4+CD25+Foxp3+ induced-Tregs and CD4+CD25−Foxp3− recovering back to resting T cells post activation.
The function of Treg cells depends on cytokine production and Foxp3 expression. Treg displays regulatory function by both cell-contact-dependent and -independent manners. In the latter mechanism, Treg secretes cytokines such as TGF-β and IL-10. TGF-β and IL-10 were reported to be essential for Treg (33-35). On the other hand, IFN-γ has also been found to play an important role in regulatory T cell differentiation, activation and activity (36,37). In the present study, we determined the levels of IFN-γ, TGF-β, and IL-10 produced by the CD4+CD25+T subset of SLE patients in comparisons with healthy controls and with the CD4+CD25− T subset. The results showed that in SLE patients, IFN-γ+ cells both in the CD4+CD25+T subset and the CD4+CD25− T subset fresh-isolated significantly increased, representing an abnormal activation of T cells. On the other hand, the IFN-γ-producing response of these cells to stimulation more noticeably decreased. This impaired ability to produce IFN-γ upon stimulation was found not only in the CD4+CD25+T subset but also in the CD4+CD25− T subset, probably representing a functional impairment in several T subsets, rather than only in the CD4+CD25+T subset. As for TGF-β-producing cells in SLE patients, they were found to be reduced in number in the CD4+CD25+T subset and the CD4+CD25− T subset. The results also showed that in SLE patients, although the CD4+ CD25+IL-10+T subset increased, the induced IL-10 expression by the CD4+CD25− T subset in response to stimulation significantly decreased. The two findings showing that the CD4+IFN-γ+T subset was defective in response to stimulation and that the CD4+IL-10+T subset increased in SLE patient, are in accordance with the results of our previous studies (38). Furthermore, here, we revealed a defect in the IL-10-producing response to stimulation of the CD4+CD25– T subset in SLE patients. However, these abnormalities in the expressions of IFN-γ, TGF-β, and IL-10 did not show any correlations with the disease activity in the present study. The CD4+CD25+ T subset, of course, is not a real Treg cell. The abnormalities in the expressions of IFN-γ, TGF-β, and IL-10 by the CD4+CD25+ T subset may give a clue for reference in studies on the abnormality of CD4+CD25+Foxp3+ Treg and its role in the pathogenesis of SLE.
Conclusions
There was a clear decrease of CD4+CD25+Foxp3+Treg in patients with active SLE, which was correlated with SLEDAI, immunological abnormalities, and types of tissue damage. The study suggests that CD4+CD25+Foxp3+Treg plays an important role in the pathogenesis of SLE and is a possible choice for an immunotherapeutic target for SLE. As for the CD4+CD25+ T subset, the deficient expression of Foxp3, rather than the abnormal expression of IFN-γ, TGF-β or IL-10, is more significant in the pathogenesis of SLE.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Research Ethics Committee of Nanjing Medical University (ID: 2017-SR-121). Informed consent was obtained from all patients and control subjects.
References
- Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 2004;22:531-62. [Crossref] [PubMed]
- Sakaguchi S. Regulatory T cells: history and perspective. Methods Mol Biol 2011;707:3-17. [Crossref] [PubMed]
- Cooles FA, Isaacs JD, Anderson AE. Treg Cells in Rheumatoid Arthritis: An Update. Curr Rheumatol Rep 2013;15:352-60. [Crossref] [PubMed]
- Moradi B, Schnatzer P, Hagmann S, et al. CD4+CD25+/highCD127low/-− regulatory T cells are enriched in rheumatoid arthritis and osteoarthritis joints--analysis of frequency and phenotype in synovial membrane, synovial fluid and peripheral blood. Arthritis Res Ther 2014;16:R97. [Crossref] [PubMed]
- Lin PP, Li QR, Zhu JS, et al. The change of CD4+CD25+Treg in the EAE mouse model transplanted with mesenchymal stem cells. Chin J Immunol 2013;10:1037-41.
- Buckner JH. Mechanisms of impaired regulation by CD4+CD25+Foxp3+ regulatory T cells in human autoimmune diseases. Nat Rev Immunol 2010;10:849-59. [Crossref] [PubMed]
- Miyara M, Ito Y, Sakaquchi S. TREG-cell therapies for autoimmune rheumatoid disease. Nat Rev Rheumatol 2014;10:543-51. [Crossref] [PubMed]
- Crispin JC, Martinez A, Alcocer-Varela J. Quantification of regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun 2003;21:273-76. [Crossref] [PubMed]
- Liu MF, Wang CR, Wu CR. Decreased CD4+CD25+T cells in peripheral blood of patients with systemic lupus erythematosus. Scand J Immunol 2004;59:198-202. [Crossref] [PubMed]
- Miyara M, Amoura Z, Parizot C, et al. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol 2005;175:8392-400. [Crossref] [PubMed]
- Alvarado-Sánchez B, Hernandez-Castro B, Portales-Perez D, et al. Regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun 2006;27:110-8. [Crossref] [PubMed]
- Valencia X, Yarboro C, Illei G, et al. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol 2007;178:2579-88. [Crossref] [PubMed]
- Bonelli M, Savitskaya A, von Dalwigk K, et al. Quantitative and qualitative deficiencies of regulatory T cells in patients with systemic lupus erythematosus (SLE). Int Immunol 2008;20:861-8. [Crossref] [PubMed]
- Yan B, Ye S, Chen G, et al. Dysfunctional CD4+, CD25+ regulatory T cells in untreated active systemic lupus erythematosus secondary to interferon-alpha-producing antigen-presenting Cells. Arthritis Rheum 2008;58:801-12. [Crossref] [PubMed]
- Bonelli M, Smolen JS, Scheinecker C. Treg and lupus. Ann Rheum Dis 2010;69:i65-6. [Crossref] [PubMed]
- Ohl K, Tenbrock K. Regulatory T cells in systemic lupus erythematosus. Eur J Immunol 2015;45:344-55. [Crossref] [PubMed]
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [Crossref] [PubMed]
- Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002;29:288-91. [PubMed]
- Zhang C, Yang X, Wang H, et al. Analysis of peripheral blood CD4+CS25+ T cells from the patients with systemic lupus erythematosus. Acta Univ Med Nanjing 2004;24:455-8.
- Mesquita D, de Melo CW, Araujo J, et al. Systemic lupus erythematosus exhibits a dynamic and continuum spectrum of effector/regulatory T cells. Scand J Rheumatol 2011;40:41-50. [Crossref] [PubMed]
- Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest 2007;117:1167-74. [Crossref] [PubMed]
- Campbell DJ, Ziegler SF. FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol 2007;7:305-10. [Crossref] [PubMed]
- Bruinsma M, van Soest PL, Leenen PJ, et al. Keratinocyte growth factor improves allogeneic bone marrow engraftment through a CD4+Foxp3+ regulatory T cell-dependent mechanism. J Immunol 2009;182:7364-9. [Crossref] [PubMed]
- Tran DQ, Anderssona J, Wangb R, et al. GARP(LRRC32) is essential for the surface expression of latent TGF-β on platelets and activated Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A 2009;106:13445-50. [Crossref] [PubMed]
- Miyara M, Gorochov G, Ehrenstein M, et al. Human Foxp3+ regulatory T cells in systemic autoimmune diseases. Autoimmun Rev 2011;10:744-55. [Crossref] [PubMed]
- Nocentini G, Alunno A, Petrillo MG, et al. Expansion of regulatory GITR+CD25low/-CD4+ T cells in systemic lupus erythematosus patients. Arthritis Res Ther 2014;16:444-58. [Crossref] [PubMed]
- Yang HX, Zhang W, Zhao LD, et al. Are CD4+CD25-Foxp3+ cells in untreated new-onset lupus patients regulatory T cells? Arthritis Res Ther 2009;11:R153. [Crossref] [PubMed]
- Zhang B, Zhang X, Tang FL, et al. Clinical significance of increased CD4+CD25-Foxp3+ T cells in patients with new-onset systemic lupus erythematosus. Ann Rheum Dis 2008;67:1037-40. [Crossref] [PubMed]
- Bonelli M, Savitskaya A, Steiner CW, et al. Phenotypic and functional analysis of CD4+ CD25-Foxp3+ T cells in patients with systemic lupus erythematosus. J Immunol 2009;182:1689-95. [Crossref] [PubMed]
- Horwitz DA. Identity of mysterious CD4+CD25-Foxp3+ cells in SLE. Arthritis Res Ther 2010;12:101. [Crossref] [PubMed]
- Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009;30:899-911. [Crossref] [PubMed]
- Huynh A, DuPage M, Priyadharshini B, et al. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nature Immunology 2015;16:188-96. [Crossref] [PubMed]
- Raphael I, Nalawade S, Eagar TN, et al. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015;74:5-17. [Crossref] [PubMed]
- Travis MA, Sheppard D. TGF-β activation and function in immunity. Annu Rev Immunol 2014;32:51-82. [Crossref] [PubMed]
- Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 2006;25:455-71. [Crossref] [PubMed]
- Nomura M, Hodgkinson SJ, Tran GT, et al. Cytokines affecting CD4+T regulatory cells in transplant tolerance. II. Interferon gamma (IFN-γ) promotes survival of alloantigen-specific CD4+T regulatory cells. Transpl Immunol 2017;42:24-33. [Crossref] [PubMed]
- Wood KJ, Sawitzki B. Interferon gamma: a crucial role in the function of induced regulatory T cells in vivo. Trends Immunol 2006;27:183-7. [Crossref] [PubMed]
- Ke Y, Wang L, Shen Y, et al. The pathogenesis significance of IFN-γ+ and IL-10+ cells in patients with systemic lupus erythematosus. J Clin Dermatol 2003;11:638-40.


