
Pulmonary metastases of fibrosarcomatous dermatofibrosarcoma protuberans respond to apatinib-based angiogenesis and chemotherapy: a case report
Introduction
Dermatofibrosarcoma protuberans (DFSP) is a rare and locally aggressive tumor arising from fibroblasts in subcutaneous mesenchymal tissue of the skin, commonly occurring in the extremities and trunk (1,2). DFSP is divided into various different subtypes. The fibrosarcomatous variant of DFSP (FS-DFSP) is a variant of DFSP and is diagnosed when more than 5% of fibrosarcomatous changes in the area are observed in typical DFSP (2). The incidence of FS-DFSP remains about 13% to 16% of DFSP (3). FS-DFSP has a higher risk of local recurrence, metastasis, and death from the disease than classical DFSP, usually with a poor outcome (2,4). Surgery and adjuvant radiotherapy are major therapeutic strategies for resectable DFSP. However, multidisciplinary consultation including chemotherapy, radiotherapy, target therapy, and anti-angiogenesis therapy is needed for metastatic diseases.
Angiogenesis is important in growth and differentiation processes of numerous malignancies, as well as in soft tissue sarcomas (STS). The signal pathways such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) are essential in angiogenesis. Most STS are insensitive to chemotherapy. Anti-angiogenesis drugs, such as bevacizumab, sunitinib, sorafenib, and pazopanib have been reported to significantly increase the antitumor efficacy when combined with chemotherapy (5-7). Regimens combining anti-angiogenesis with chemotherapy would, therefore, be worth exploring (5,8).
Apatinib, a small molecule tyrosine kinase inhibitor (TKI) targeting vascular endothelial growth factor receptor-2 (VEGFR-2), has been approved by the China Food and Drug Administration (CFDA) for the treatment of metastatic or advanced gastric cancer as a third line or more chemotherapy. Apatinib has preliminarily shown promising efficacy and safety in STS (6,7,9-11). However, there is no report of apatinib for metastatic DFSP.
Here, we report an obstinate case of FS-DFSP arising from the orbit which relapsed and metastasized repeatedly after multiple surgeries and adjuvant radiotherapy. However, the patient responded to apatinib combined with chemotherapy, indicating apatinib may be a potential therapeutic option for metastatic DFSP.
Case presentation
A 29-year-old female Chinese judo athlete initially presented with a subcutaneous indolent-growth lesion in the left orbit, which had been protruding since December 2011. The mass was excised in March 2013, and FS-DFSP was diagnosed by H&E and immunohistochemical staining.
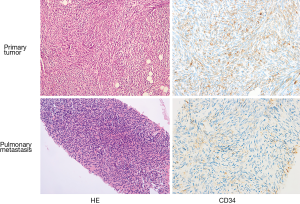
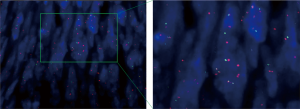
Unfortunately, the tumor recurred at the same site just five months later in August 2013. A second surgical excision was performed and followed by adjuvant radiation on the forehead with a dose of 60 Gy/25 f. The tumor recurred repeatedly afterward. Between March 2013 and January 2017, the patient received eleven resections with negative surgical margins and subsequent reconstructions, with extensive excision of adjacent tissues, including exenteration of the left eyeball. Everything went well until December 2016 when she felt intermittent shortness of breath and coughed. Bilateral lung metastases were diagnosed by computed tomography (CT) and sequential percutaneous lung biopsy in May 2017. H&E and immunohistochemical staining showed positive staining with CD34 (Figure 1), and fluorescence in situ hybridization (FISH) test revealed chromosomal translocation (17; 22) (q21; q13), which suggested the occurrence of fusion gene COL1A1-PDGFB (Figure 2). Therefore, FS-DFSP was pathologically diagnosed, consistent with the primary tumor.
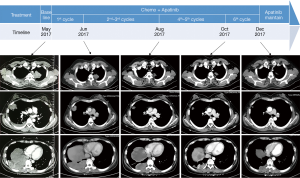
The patient refused to receive imatinib due to excessive expense of the targeted medication. She then received combined chemotherapy with ifosfamide, cisplatin and apatinib (ifosfamide 1,600 mg/m2 intravenously on days 1 to 5, cisplatin 25 mg/m2 intravenously on days 1 to 3, and apatinib 500 mg daily oral administration on days 1 to 14, every 3 weeks). After 6 courses of treatment she felt relieved from her shortness of breath and cough; a chest CT scan showed that a remarkable partial response was achieved (Figure 3). Later, maintenance therapy with apatinib alone (500 mg, daily oral administration on days 1 to 14, every 3 weeks) was given. The patient is under a stable condition at the time of writing.
Discussion
DFSP is considered a rare, indolent-growth malignancy, which recurs easily with fewer metastases (1). When compared to DFSP, FS-DFSP carries a greater risk of local recurrence, metastasis, and death from disease (29.8% vs. 13.7%, 14.4% vs. 1.1%, 14.1% vs. 0.8%, respectively) (2). DFSP is refractory, and the treatment is limited. Complete surgical excision with adjuvant radiotherapy is the cornerstone of therapy for localized DFSP. Locally recurrent DFSP can be salvaged by further resection. However, for FS-DFSP and metastasis DFSP, the treatment standard is unclear and multidisciplinary consultation is recommended. Chemotherapy, target therapy, and anti-angiogenesis therapy are common treatment options.
Anti-angiogenesis is an important therapeutic strategy, and a few reports have shown that it may play a potential role in DFSP. Numerous anti-angiogenic drugs, such as bevacizumab, sunitinib, sorafenib, pazopanib, and apatinib have already shown potential efficacy and safety in STS, with a single agent or in combination with chemotherapy (5-7,9-11). Only four anti-angiogenesis studies were reported for DFSP previously, including treatment with sunitinib, sorafenib or pazopanib (12-15), but with no cases with apatinib treatment yet reported (Table 1).

Full table
Most common toxicities of apatinib from clinical trials of gastric cancer are a hand-foot syndrome, proteinuria, and hypertension with an incidence of 27.8%, 47.7%, and 35.2%, respectively (16). Combination apatinib with chemotherapy may increase side-effects, and dose reduction of apatinib presents good tolerance with considerable antiangiogenic effect (17). In this case, the patient was given a reduced dose of apatinib and showed good tolerance to it, indicating that a reduced dose of apatinib may be a reasonable strategy when combined with chemotherapy. As far as we know, this is the first report of a case where metastatic FS-DFSP responded to chemotherapy plus apatinib and achieved a significant response.
However, how much of a role apatinib played in the successful treatment has yet to be determined, because apatinib was combined with chemotherapy. A case report demonstrated that ifosfamide as first-line chemotherapy is useless for metastatic DFSP (18). A review also argued that conventional chemotherapy plays little part in the management of inoperable or metastatic DFSP (19). A study showed that combination therapy with VEGFR inhibitor and chemotherapy achieved greater clinical benefit than conventional therapy for STS (20). Therefore, based on the good response in this report and the previous related literature, we speculate that apatinib might play a positive role in the treatment.
Platelet-derived growth factor receptor (PDGFR) is an important driver gene in DFSP with genetic translocations. Sunitinib, sorafenib, and pazopanib are all molecular multi-targeted TKIs, including PDGFR and VEGFR, so the anti-angiogenesis efficacy may be biased by the inhibiting role of PDGFR pathway. However, apatinib only targeting VEGFR is more representative of anti-angiogenesis medication than sunitinib or pazopanib. Apatinib and pazopanib, which have been reported to be effective in DFSP, mostly inhibit the VEGF pathway. Therefore, this case indicated that the VEGF pathway might be important in the development and progression of DFSP. In view of this, we hypothesize that anti-VEGF-pathway may be a potential treatment for cases resistant to imatinib or cases without genetic translocations in DFSP.
In conclusion, this report provides clinical evidence that apatinib combined with chemotherapy may be a potential treatment option for DFSP. However, this notion still warrants investigation in larger, prospective clinical trials.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation of China (Grant No. 81402561).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Saiag P, Grob JJ, Lebbe C, et al. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. Eur J Cancer 2015;51:2604-8. [Crossref] [PubMed]
- Liang CA, Jambusaria-Pahlajani A, Karia PS, et al. A systematic review of outcome data for dermatofibrosarcoma protuberans with and without fibrosarcomatous change. J Am Acad Dermatol 2014;71:781-6. [Crossref] [PubMed]
- Cai H, Wang Y, Wu J, et al. Dermatofibrosarcoma protuberans: clinical diagnoses and treatment results of 260 cases in China. J Surg Oncol 2012;105:142-8. [Crossref] [PubMed]
- Abbott JJ, Oliveira AM, Nascimento AG. The prognostic significance of fibrosarcomatous transformation in dermatofibrosarcoma protuberans. Am J Surg Pathol 2006;30:436-43. [Crossref] [PubMed]
- Martin-Liberal J, Judson I, Benson C. Antiangiogenic approach in soft-tissue sarcomas. Expert Rev Anticancer Ther 2013;13:975-82. [Crossref] [PubMed]
- Kawai A, Araki N, Hiraga H, et al. A randomized, double-blind, placebo-controlled, Phase III study of pazopanib in patients with soft tissue sarcoma: results from the Japanese subgroup. Jpn J Clin Oncol 2016;46:248-53. [Crossref] [PubMed]
- van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879-86. [Crossref] [PubMed]
- Erber R, Thurnher A, Katsen AD, et al. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. Faseb J 2004;18:338-40. [Crossref] [PubMed]
- Dong M, Bi J, Liu X, et al. Significant partial response of metastatic intra-abdominal and pelvic round cell liposarcoma to a small-molecule VEGFR-2 tyrosine kinase inhibitor apatinib: A case report. Medicine (Baltimore) 2016;95:e4368. [Crossref] [PubMed]
- Zhu B, Li J, Xie Q, et al. Efficacy and safety of apatinib monotherapy in advanced bone and soft tissue sarcoma: An observational study. Cancer Biol Ther 2018;19:198-204. [Crossref] [PubMed]
- Li F, Liao Z, Zhao J, et al. Efficacy and safety of Apatinib in stage IV sarcomas: experience of a major sarcoma center in China. Oncotarget 2017;8:64471-80. [PubMed]
- Fu Y, Kang H, Zhao H, et al. Sunitinib for patients with locally advanced or distantly metastatic dermatofibrosarcoma protuberans but resistant to imatinib. Int J Clin Exp Med 2015;8:8288-94. [PubMed]
- Kamar FG, Kairouz VF, Sabri AN. Dermatofibrosarcoma protuberans (DFSP) successfully treated with sorafenib: case report. Clin Sarcoma Res 2013;3:5. [Crossref] [PubMed]
- Miyagawa T, Kadono T, Kimura T, et al. Pazopanib induced a partial response in a patient with metastatic fibrosarcomatous dermatofibrosarcoma protuberans without genetic translocations resistant to mesna, doxorubicin, ifosfamide and dacarbazine chemotherapy and gemcitabine-docetaxel chemotherapy. J Dermatol 2017;44:e21-2. [Crossref] [PubMed]
- Ong HS, Ji T, Wang LZ, et al. Dermatofibrosarcoma protuberans on the right neck with superior vena cava syndrome: case report and literature review. Int J Oral Maxillofac Surg 2013;42:707-10. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol 2016;34:1448-54. [Crossref] [PubMed]
- Wu F, Zhang S, Gao G, et al. Successful treatment using apatinib with or without docetaxel in heavily pretreated advanced non-squamous non-small cell lung cancer: A case report and literature review. Cancer Biology & Therapy 2018;19:141-4. [Crossref] [PubMed]
- Labropoulos SV, Fletcher JA, Oliveira AM, et al. Sustained complete remission of metastatic dermatofibrosarcoma protuberans with imatinib mesylate. Anticancer Drugs 2005;16:461-6. [Crossref] [PubMed]
- Constantinidou A, Pollack S, Loggers E, et al. The evolution of systemic therapy in sarcoma. Expert Rev Anticancer Ther 2013;13:211-23. [Crossref] [PubMed]
- García del Muro X, Maurel J, Martínez Trufero J, et al. Phase II trial of ifosfamide in combination with the VEGFR inhibitor sorafenib in advanced soft tissue sarcoma: a Spanish group for research on sarcomas (GEIS) study. Investigational New Drugs 2018;36:468-75. [Crossref] [PubMed]


