Risk factors associated with prolonged air leak after video-assisted thoracic surgery pulmonary resection: a predictive model and meta-analysis
Introduction
Prolonged air leak (PAL) is a condition where air escapes from the lung parenchyma into the pleural space for more than 5 days after lung surgery (1). PALs are considered to be the most common postoperative complication of the lung surgery with an incidence of 6–26% (2-17). Previous studies have found that PAL is associated with other pulmonary complications such as atelectasis and pneumonia, which often translate into longer hospital stays and more hospitalization costs (18,19). In recent years, the enhanced recovery pathway after surgery (ERAS) has become a focus of surgery as a perioperative program to speed up patient recovery, was shown to reduce potential complications and decrease the length of hospital stays (20,21). The Italian ERAS Group (21) recommended that air leak prevention was a crucial part of ERAS, and certain measures, such as pleural tent, surgical sealant, staple-line reinforcement, should be taken in high-risk patients (e.g., those with severe emphysema or intraoperative air leak). This study contributes to research on the risk factors for PAL after video-assisted thoracic surgery (VATS) by conducting a retrospective, case-control study and providing a literature review and the first meta-analysis of published clinical data on this topic.
Methods
Data source and patient selection
Consecutive medical records of consecutive patients who underwent major pulmonary resection (lobectomy, sleeve lobectomy and segmentectomy) between January 2015 and August 2017 at the Department of Thoracic Surgery, Xiangya Hospital, Central South University were retrospectively collected. This project was reviewed and approved by the Ethics Committee of the Xiangya Hospital, Central South University, and informed consent was waived as it was a retrospective study (IRB number: 2017121009). Three individuals were excluded from this study: patients who underwent thoracotomy or pneumonectomy, patients with postoperative bronchopleural fistula, and patients who died before chest tube removal. PAL was defined as an air leak persisting for more than 5 days after lung surgery.
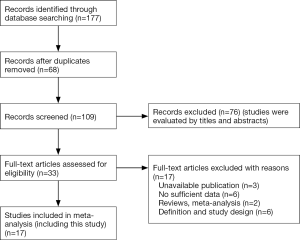
The meta-analysis part of this research conformed to the guidelines set out in the PRISMA Statement. We systematically searched for relevant studies indexed in PubMed, Embase and Cochrane Library. The search dates ranged from January 1, 1984 to November 1, 2017. The following combinations of terms were used: “PAL or prolonged air leak OR persistent air leak”, “risk factor OR predict*”, “pulmonary resection OR lung resection OR pulmonary lobectomy” (Table S1). We also scrutinized previous reviews for additional studies. For studies with incomplete data, we tried to contact the corresponding author to obtain further details. The inclusion criteria were: (I) case-control studies related to risk factors of PAL after lung resection and (II) analysis of air leaks at least 5 days in duration. The exclusion criteria were: (I) studies without sufficient data; (II) reviews or meta-analysis; (III) low-quality publications with an unrepresentative cohort or inadequate methods (Figure S1). Two investigators (H Pan and Y Cheng) independently scrutinized the final included studies.

Full table
Surgical protocol
The most common anesthesia strategies included of general anesthesia, a central venous catheter, or double-lumen tube. Systemic lymph node dissection was performed for lung cancer in all studies. Two types of mechanical staplers were used to close incomplete fissures (The ECHELON FLEX™ GST System or Endo GIA™ Tri-Staple™). After lung resection, a lung inflation test was performed to determine the presence of a significant air leak; any leaks were repaired by suturing. No biological glue, hemostatic gauze, or other materials were used during surgery. Additionally, one or two 28-Fr chest tubes were then placed after surgery. The indications for the removal of chest tube included: no leakage, drainage volume less than 100–200 mL/24 h (or 2–4 mL/kg/24 h). According to Cerfolio classification of air leaks (22,23), assessment of air leak are classified in 4 grade. If there was an air leak greater than forced expiratory (FE) leak on the classification system after surgery, biologic sealants were routinely used for treatment.
Data collection
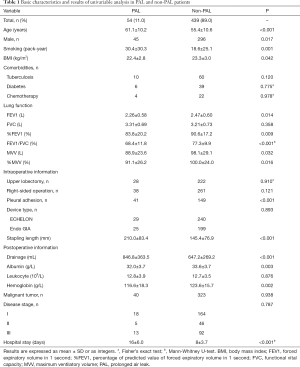
Several clinical data were extracted from the included studies: basic patient characteristics, pulmonary function test, intraoperative factors, postoperative factors, postoperative pathology and malignant tumor stage (Table 1). The extent of pleural adhesion was obtained from the surgical records and judged directly by the surgeon. Early postoperative drainage was defined as the sum of pleural fluid for the 3 days after surgery. Stapling length was calculated by the number of staples multiplied by the length of a staple.
Full table
Two investigators (H Pan and Y Cheng) extracted the data from the eligible studies independently. Disagreements between investigators were resolved by discussion with the research team. Odds ratios (OR) with associated 95% confidence intervals (CIs) of each risk factor were extracted from eligible studies. Finally, study quality was assessed by two investigators (Table S2).

Full table
Statistical analysis
Categorical variables were compared by either the χ2 test or Fisher’s exact test, while continuous variables were compared using the t-test or Mann-Whitney U-test. Receiver operating characteristic (ROC) curve were used to obtain cutoff values and areas under the ROC curves (AUC). Variables with less than a 0.05 significance level in the univariate analysis were entered into the logistic regression for multivariate analysis. Continuous variables were converted into binary variables based on their respective cutoff values. All data were analyzed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA). According to their contribution, the corresponding variables of each factor were analyzed in the regression model and a nomogram point scoring system was built as a predictive model. A concordance index (C-index) and the calibration curve were constructed to measure the performance of the nomogram. R version 3.4.3 (http://www.r-project.org/) was used to construct the nomogram.
For binary variables, we calculated pooled ORs and 95% CIs; pooled weighted mean differences (WMDs) were calculated for continuous variables. Each risk factor in the meta-analysis must have been evaluated in at least three included studies. We used the Q-test and I2 index (24) to assess the heterogeneity. We then assessed publication bias with Egger’s test. Subgroup analyses were performed to identify heterogeneity. The meta-analysis was completed with StataSE version 12.0 (Stata Corp., Texas, USA).
All statistical tests were two-sided, and a P value of <0.05 was considered to be statistically significance.
Results
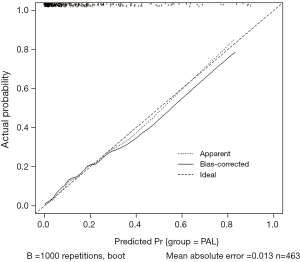
A total of 493 patients were included in this study. Basic characteristics and univariate analysis are shown in Table 1. The occurrence of PAL was 11.0% (54/493), patients with complication of PAL had significantly longer postoperative hospital stays (16±6.0 vs. 8±3.7, P<0.001). Age, gender, smoking, BMI, pulmonary function, pleural adhesion, stapling length, drainage, albumin, hemoglobin also showed significant statistical differences. Since males accounted for 96% of smoking patients (226/235), we reanalyzed the effect on PAL for smoking male patients (P=0.044). For this reason, the gender was not included in the later multivariate analyses. ROC curve indicated that forced the first second of expiratory volume/forced vital capacity (FEV1/FVC) was an optimal variable of pulmonary function (AUC =0.72, Table S3). Excluding cases of missing information, a total of 463 patients were included in the logistic regression model. The results of regression analysis are shown in Table 2. Multivariate analyses indicated that age, smoking, FEV1/FVC, pleural adhesion, stapling length and drainage were factors significantly associated with PAL. All independent factors analyzed in this study and three factors found significant in previous study (BMI, smoking, right-side operation) were integrated into the construction of a nomogram (Figure 1). The C-index for PAL prediction was 0.858 and the calibration curve showed good consistency between prediction and observation (Figure 2).
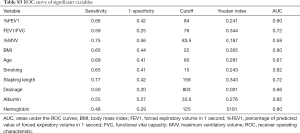
Full table
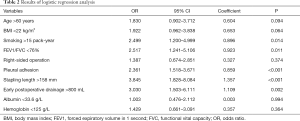
Full table
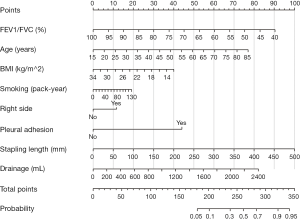
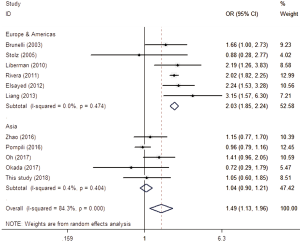
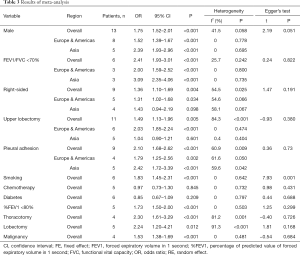
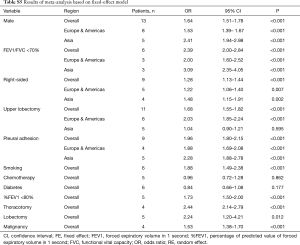
After searching and screening, there were 17 eligible studies for meta-analysis, including 2 multicenter database analyses, 2 prospective studies and 13 retrospective studies (Table S4). Variability in study design and risk of bias may lead to substantial heterogeneity. Therefore, the results of meta-analysis were shown in Table 3, which were based on random-effects model. Fixed-effect meta-analysis were presented in Table S5. We calculated pooled unadjusted ORs for 12 risk factors. Factors included male, smoking, %FEV1, FEV1/FVC, and malignancy had statistical significance and heterogeneity was acceptable (I2 =0–41.5%). To avoid studies with large sample sizes from dominate the overall results, we re-analyzed the corresponding factors after removing Rivera’s (7) study. The results were not significantly changed, except for the obvious difference with regards to the right-sided operation factor. Egger’s test did not indicate any significant publication bias in this analysis. Subgroup analyses stratified by region showed that upper lobectomy was a risk factor for PAL in Europe and America, but not in Asia. Furthermore, in subgroup analysis by region heterogeneity disappeared (Figure 3). Subgroup analyses of other factors were consistent with the overall results (Figure 4).

Full table
Full table
Full table
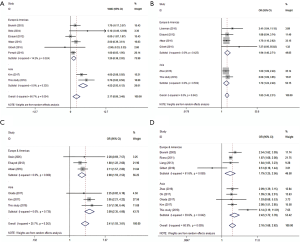
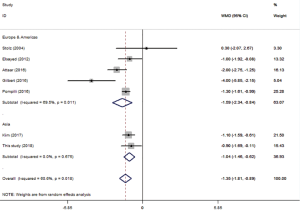
Age and BMI were analyzed using pooled WMD. As part of age subgroup analysis (Figure 4A), 8 studies were included to yield a pooled WMD of 2.17 (P=0.004, 95% CI: 0.86–3.48, I2 =66.7%). In BMI analysis (Figure S2), 7 studies were included and pooled WMD was −1.35 (P<0.001, 95% CI: −1.81 to −0.89, I2 =60.6%). Subgroup analysis did not reduce the heterogeneity, but consistency was reflected in the forest plot.
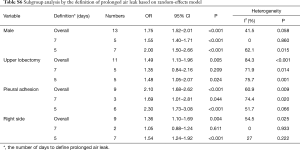
In subgroup analysis using the definition of PAL, heterogeneity did not decrease and there was no difference in results between subgroups (Table S6).
Full table
Discussion
The risk factors of PAL after lung resection have been the subject of previous study, with many prediction models proposed. However, the relevant independent risk factors in such a clinical situation remain controversial. Pre- and intra-operative identification of high-risk patients contributes the success of intraoperative interventions such as pleural tenting, prophylactic pneumoperitoneum, sealing material and the buttressing of staple lines (25). However, few studies have examined postoperative risk factors of PAL which could help determine interventional indications. There may be yet undiscovered associations and geographical differences among various clinical factors and PAL.
Consistent with previous study (4,7,10,11,14,16), we found that poor pulmonary function, smoking, intraoperative pleural adhesion were all risk factors for PAL. Additionally, three variables of pulmonary function (%FEV1, FEV1/FVC, %MVV) were found to be potential predictors in preliminary analysis. Using the ROC curve method, we found that, unlike previous studies, FEV1/FVC had better identification ability than %FEV1 (AUC =0.72). A possible explanation for this finding might be that patients undergoing lobectomy required acceptable pulmonary function. In our study, 99% (407/412) of lobectomy patients had %FEV1 >50%. Therefore FEV1/FVC could be indicative of airflow obstruction and elastic recoil pressure (26,27). According to the result of meta-analysis, pulmonary function was the most consistent risk factor for postoperative PAL.
Meta-analysis also showed that patients with the older age, male gender, and low BMI were more likely to have PAL. In China, males account for 90.1% of all smokers in the general population (28), and this proportion was up to 96% in our center; hence, gender variable was not included in our regression analysis. Additionally, age and BMI showed a non-significant trend in our regression analysis (P=0.094, 0.064, respectively). Pulmonary function results were adjusted for age as they are representative. Low BMI may imply poor nutritional status and obesity was found to be protective against PAL, which was shown to be due to a variation in respiratory rates and tidal volumes in obese patients (10).
We identified upper lobectomy as a risk factor for PAL. Interestingly, subgroup analysis indicated that this surgical procedure was of significance in European and North American studies but not in Asian ones. This difference might be due to differences in thorax morphology and pulmonary anatomy among ethnic groups. Researchers believe that residual pleural spaces with incomplete visceral-parietal apposition in the upper thoracic cavity account for air leakage after upper lobectomy (1); however, this cannot completely explain our results. Currently there is no research to confirm this interpretation and thus should be an area of interest in the future.
Extensive adhesions increase parenchymal injury during surgery. However, as this factor is assessed by subjective judgment, the literature remains inconsistent. Clearly on the forest plot (Figure 4D), Gilbert’s (11) result was the main source of heterogeneity, as the other seven studies showed good consistency. Pleural adhesion may be a good predictor of postoperative PAL when strictly defined. Lobectomy, right-side operation, malignancy and thoracotomy were also found to be significant upon analysis, but were not convincing enough, because of the obvious heterogeneity or the limited number of studies.
Moreover, pulmonary fissure was another risk factor discussed in past (5,14), but no accurate measurement of such a complication has been proposed. To control for bias, we calculated the stapling length as an objective measurement for incomplete fissures of lung. We found that stapling length was an independent risk factor for PAL. Future studies should take steps to be scrutinize this factor.
Postoperative factors could be assessed for the timing of intervention after air leak. Okada et al. (12) and Oh et al. (16) emphasized the importance of quantifying air leakage in the postoperative period. Postoperative albumin, leukocyte, hemoglobin and three days postoperative drainage was not previously considered clinically relevant. However, our data indicated that a large amount of early postoperative drainage was related to the occurrence of PAL, presumably because more pleural effusion would hamper the healing of alveolar fistula and the formation of pleural adhesions.
Several predictive models have been proposed for PAL, including scoring systems (6,11,15,29) based on two or three risk factors (e.g., %FEV1, male, BMI, pleural adhesions, smoking) which could be further divided into demographic features and pulmonary function. However, the predictive power of these models has been unsatisfactory. At present, nomograms have been applied widely and have proven to be more accurate than the conventional scoring systems (30). Attaar et al. (10) derived a nomogram for PAL with good discriminatory accuracy in 2016, but they ignored postoperative factors. In contrast, we drew a nomogram based on the significant variables in multivariate analysis and, while using the previous risk models from Attaar et al. (10) and Gilbert et al. (11) as references, right-side operation and BMI variables were also included. Our nomogram, with 86% discriminatory accuracy and a calibration curve close to the ideal, had better predictive value than previous studies. Notably, we were not able to validate other models using our data because of significant differences in baseline characteristics. Application of a predictive model widely or across geographical regions should be done cautiously before a multi-center, large sample size study come out.
This study provided the first meta-analysis to identify the relationship between various factors and PAL. Subgroup analysis was performed to minimize heterogeneity, as well as to explore regional differences in risk factors. VATS has been widely accepted as part of thoracic surgery because it is minimally invasion and requires smaller incisions (31,32). Unfortunately, the difference between thoracotomy and VATS was not analyzed in our meta-analysis because only four studies focused on VATS. Nonetheless, our results involving thoracotomy and VATS were meaningful and convincing, even with some heterogeneity.
There are limitations to our study that should be noted. First, in order to minimize the differences in baseline characteristics, we did not analyze all types of pulmonary surgery (lung volume reduction surgery and bulla resections were excluded). Secondly, our prediction model requires prospective external validation, which will be done as part of our follow-up research. Finally, only a few studies were included in the meta-analysis for some factors (e.g., lobectomy, chemotherapy, malignancy) and detailed data was unavailable in some studies.
Conclusions
Taken together, in order to speed patient recovery and decrease hospital stay lengths, preoperative, intraoperative, and postoperative factors should be taken into account and analyzed for prevention and early treatment of PAL after VATS lung resection. Researchers should also pay close attention to the differences in surgical risk factors among ethnic groups when using a predictive model.
Acknowledgements
Funding: This study was supported by: Programs of Changsha Science and Technology Agent (kq1701080); Natural Science Foundation General Program of Hunan Province (2017JJ2386); Clinic and Rehabilitation research foundation of Sinobioway group (xywm2015I37); National Natural Science Youth Foundation of China (81602027, 81702928); Key Research and Development Program of Hunan Province (2016JC2039).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This project was reviewed and approved by the Ethics Committee of the Xiangya Hospital, Central South University (IRB number: 2017121009), and informed consent was waived as it was a retrospective study.
References
- Brunelli A, Cassivi SD, Halgren L. Risk factors for prolonged air leak after pulmonary resection. Thorac Surg Clin 2010;20:359-64. [Crossref] [PubMed]
- Abolhoda A, Liu D, Brooks A, et al. Prolonged air leak following radical upper lobectomy: an analysis of incidence and possible risk factors. Chest 1998;113:1507-10. [Crossref] [PubMed]
- Brunelli A, Monteverde M, Borri A, et al. Predictors of prolonged air leak after pulmonary lobectomy. Ann Thorac Surg 2004;77:1205-10; discussion 1210. [Crossref] [PubMed]
- Liberman M, Muzikansky A, Wright CD, et al. Incidence and risk factors of persistent air leak after major pulmonary resection and use of chemical pleurodesis. Ann Thorac Surg 2010;89:891-7; discussion 897-8. [Crossref] [PubMed]
- Petrella F, Rizzo S, Radice D, et al. Predicting prolonged air leak after standard pulmonary lobectomy: computed tomography assessment and risk factors stratification. Surgeon 2011;9:72-7. [Crossref] [PubMed]
- Lee L, Hanley SC, Robineau C, et al. Estimating the risk of prolonged air leak after pulmonary resection using a simple scoring system. J Am Coll Surg 2011;212:1027-32. [Crossref] [PubMed]
- Rivera C, Bernard A, Falcoz PE, et al. Characterization and prediction of prolonged air leak after pulmonary resection: a nationwide study setting up the index of prolonged air leak. Ann Thorac Surg 2011;92:1062-8; discussion 1068. [Crossref] [PubMed]
- Elsayed H, McShane J, Shackcloth M. Air leaks following pulmonary resection for lung cancer: is it a patient or surgeon related problem? Ann R Coll Surg Engl 2012;94:422-7. [Crossref] [PubMed]
- Liang S, Ivanovic J, Gilbert S, et al. Quantifying the incidence and impact of postoperative prolonged alveolar air leak after pulmonary resection. J Thorac Cardiovasc Surg 2013;145:948-54. [Crossref] [PubMed]
- Attaar A, Winger DG, Luketich JD, et al. A clinical prediction model for prolonged air leak after pulmonary resection. J Thorac Cardiovasc Surg 2017;153:690-9.e2. [Crossref] [PubMed]
- Gilbert S, Maghera S, Seely AJ, et al. Identifying Patients at Higher Risk of Prolonged Air Leak After Lung Resection. Ann Thorac Surg 2016;102:1674-9. [Crossref] [PubMed]
- Okada S, Shimada J, Kato D, et al. Prolonged air leak following lobectomy can be predicted in lung cancer patients. Surg Today 2017;47:973-9. [Crossref] [PubMed]
- Stolz AJ, Schutzner J, Lischke R, et al. Predictors of prolonged air leak following pulmonary lobectomy. Eur J Cardiothorac Surg 2005;27:334-6. [Crossref] [PubMed]
- Zhao K, Mei J, Xia C, et al. Prolonged air leak after video-assisted thoracic surgery lung cancer resection: risk factors and its effect on postoperative clinical recovery. J Thorac Dis 2017;9:1219-25. [Crossref] [PubMed]
- Pompili C, Falcoz PE, Salati M, et al. A risk score to predict the incidence of prolonged air leak after video-assisted thoracoscopic lobectomy: An analysis from the European Society of Thoracic Surgeons database. J Thorac Cardiovasc Surg 2017;153:957-65. [Crossref] [PubMed]
- Oh SG, Jung Y, Jheon S, et al. Postoperative air leak grading is useful to predict prolonged air leak after pulmonary lobectomy. J Cardiothorac Surg 2017;12:1. [Crossref] [PubMed]
- Kim WH, Lee HC, Ryu HG, et al. Intraoperative ventilatory leak predicts prolonged air leak after lung resection: A retrospective observational study. 2017;12:e0187598.
- Stephan F, Boucheseiche S, Hollande J, et al. Pulmonary complications following lung resection: a comprehensive analysis of incidence and possible risk factors. Chest 2000;118:1263-70. [Crossref] [PubMed]
- DeCamp MM, Blackstone EH, Naunheim KS, et al. Patient and surgical factors influencing air leak after lung volume reduction surgery: lessons learned from the National Emphysema Treatment Trial. Ann Thorac Surg 2006;82:197-206; discussion 207. [Crossref] [PubMed]
- Li M, Zhang J, Gan TJ, et al. Enhanced recovery after surgery pathway for patients undergoing cardiac surgery: a randomized clinical trial. Eur J Cardiothorac Surg 2018;54:491-7. [Crossref] [PubMed]
- Gonfiotti A, Viggiano D, Voltolini L, et al. Enhanced recovery after surgery and video-assisted thoracic surgery lobectomy: the Italian VATS Group surgical protocol. J Thorac Dis 2018;10:S564-70. [Crossref] [PubMed]
- Cerfolio RJ. Recent advances in the treatment of air leaks. Curr Opin Pulm Med 2005;11:319-23. [Crossref] [PubMed]
- Cerfolio RJ, Bass C, Katholi CR. Prospective randomized trial compares suction versus water seal for air leaks. Ann Thorac Surg 2001;71:1613-7. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Bmj 2003;327:557-60. [Crossref] [PubMed]
- Varela G, Jimenez MF, Novoa N, et al. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur J Cardiothorac Surg 2005;27:329-33. [Crossref] [PubMed]
- Brusasco V, Martinez F. Chronic obstructive pulmonary disease. Compr Physiol 2014;4:1-31. [PubMed]
- Xu G, Chen Z, Cao X, et al. Analysis of pulmonary function test results in a health check-up population. J Thorac Dis 2015;7:1624-9. [PubMed]
- Yang T, Jiang S, Barnett R, et al. Who smokes in smoke-free public places in China? Findings from a 21 city survey. Health Educ Res 2016;31:36-47. [PubMed]
- Brunelli A, Varela G, Refai M, et al. A scoring system to predict the risk of prolonged air leak after lobectomy. Ann Thorac Surg 2010;90:204-9. [Crossref] [PubMed]
- Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013;31:1188-95. [Crossref] [PubMed]
- Liu Y, Gao Y, Zhang H, et al. Video-assisted versus conventional thoracotomy pneumonectomy: a comparison of perioperative outcomes and short-term measures of convalescence. J Thorac Dis 2016;8:3537-42. [Crossref] [PubMed]
- Tan Y, Lv L, Duan T, et al. Comparison between submucosal tunneling endoscopic resection and video-assisted thoracoscopic surgery for large esophageal leiomyoma originating from the muscularis propria layer. Surg Endosc 2016;30:3121-7. [Crossref] [PubMed]