Quality in practice: implementation of a clinical outcomes registry in regenerative medicine
Introduction
The field of regenerative medicine is undergoing increasing interest, growth in clinical use, and stakeholders are shifting to a patient-centred treatment approach (1). In this context, the value or quality of treatment is determined when the outcomes and benefits outweigh the harm and costs (2); however, determining the clinical value of orthobiologic treatments, or injections consisting of cells, and or proteins collected from the patient, processed, and then used as treatment for underlying orthopaedic pathology (3), can prove difficult due to the limitations in a rapidly-evolving field. Therefore, there has been a great effort by stakeholders to increase available evidence, based on clinical outcomes to provide a potential road map on delivering quality for facilities providing orthobiologic therapies. Additionally, to provide ‘real-world’ feedback on safety and efficacy of these treatments, observational practice registries have emerged in these clinical environments throughout the world (4).
A patient registry is defined by Gliklich et al. (5) as “an organized system that uses observational study methods to collect uniform data (clinical and other) to evaluate specified outcomes for a population defined by a particular disease, condition or exposure, and that serves a predetermined scientific, clinical or policy purpose(s).” Various orthobiologic treatments, such as platelet-rich plasma (PRP), have increased in interest and clinical use throughout the international medical community (1). Physicians utilizing these techniques occasionally monitor the treatment outcomes through both clinical outcomes (6) and patient reported outcomes (PROs) (7,8). Tracking treatment outcomes optimizes healthcare value by demonstrating care at a lower cost (9,10) or removing non-useful treatments (4). The feedback provided through registry data can aid in the development of shared baselines (guidelines) for physicians utilizing those specific treatments (11). In contrast, trials of therapies using a reductionist framework (assessing the effect of one parameter at a time) require a much longer turnaround time before useful information is released into the public domain (12).
Currently, public/national registries and private/industry registries fall short of the individualized focus required for personalized, specific delivery of orthobiologic treatment platforms either because they do not cater to cellular based therapies, or such therapies are not the primary focus with more attention on medicinal, or operative therapies (13). Although many public/national registries focus on a specific disease or disorder, and usually have a high participation rate as they include the majority of a nation’s diverse population, but some may lack local specificity to selected population that may result in not being able to apply findings to separate, diverse populations (14). The advantage of collecting patient outcomes for cellular based products at the clinical level thus provides an effective feedback tool for doctors examining the effects of orthobiologic treatment, based on ‘real life’ data that is reflective of the population being treated (12).
A clinical outcomes registry is an organised system for collecting patient outcomes as data points relevant to the effect of treatment on pathology or injury. A key benefit of this type of registry is its agility. The registry can quickly identify underperforming products/treatments in use for a specific facility by linking the product use/technique to PROs and adverse events, which is especially useful in the field of orthobiologics as data and information about the efficacy of treatments is growing, but not fully known (6,15,16). Therefore, the PROs (17) can be used in conjunction with costs to compare [using cost-benefit analysis such as quality-adjustment life year (QALY)] and assign value to treatments (2,18). The focused nature of the registry enables a rapid treatment feedback loop which allows a clinic to make adjustments to treatment plans as needed. Additionally, the registry is more agile with providing feedback for the general patient flow or process, and allows for feedback about the recovery process, that is under reported in average literature (10). Moreover, with a clinical outcomes registry there is no need to wait for slow output/publications that are commonly associated with larger public/national and private/industry registries (11).
The clinical outcomes registry provides localized data about the population using the direct treatments/products of the facility. Thus, the facility can make informed decisions about how to improve treatments based on their specific patient flow rather than inferring from results derived from a population sample.
For cellular-based registries, treatments can be organized into four categories: (I) non-operative and non-interventional; (II) non-operative and interventional; (III) operative and non-interventional; and (IV) operative and interventional (e.g., surgical injections) (1). Most private/industry-funded registries focus on operative and non-interventional therapies and are potentially under-developed on non-operative intervention (19). Facilities using biologic therapies to treat musculoskeletal disorders need comprehensive descriptions of treatment outcomes for each category (2,18), yet other registries do this in a general way within pre-set boundaries and descriptions that lack specificity about the intervention (11). The clinical outcomes registry model consists of well characterized products administered that are specific to the facility, in conjunction with adaptable processes to alter treatment pathways in response to unfavourable patient responses.
In the context of the emerging areas of patient registries in routine clinical practice in combination with regenerative medicine, the aim of this didactic article was to describe the implementation of a clinical outcomes registry within a clinical setting for regenerative medicine.
Local problem
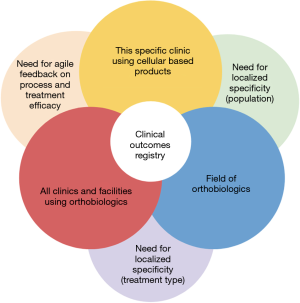
The facility in question had two overarching quality issues to be solved through the implementation of a clinical outcomes registry, including the lack of agility for treatment and process feedback and the lack of specificity regarding clinic population and treatment strategies (Figure 1).
The facility lacked an efficient system to incorporate treatment and process feedback into the facility’s patient flow and treatment plans. Without general feedback regarding the various product efficacy, systematic adjustments to treatment plans could not be made for various musculoskeletal disorders, or done so based solely on the intuition of providers. By utilizing feedback to eliminate ineffective treatments for specific disorders, then the costs of care decrease (as seen with Intermountain’s work to eliminate ineffective treatments which lowered the costs of overall healthcare) while benefits increase and in turn increase the value of specific treatment plans (11). Similarly, clinics and facilities using cellular based products and regenerative medicine can benefit from a clinical outcomes registry which would allow for more agile feedback (2,17,18) and an improved ability to create and improve effective treatment protocols.
Although large, public/national and private/industry registries provide useful evidence about patient outcomes for various treatments, they lack specificity to the clinic’s population (14) and treatment strategies (13). Facilities utilizing innovative orthobiologic products and treatments require patient outcome data specific to their population that is being treated to be comprehensive in their care delivery. Moreover, specificity regarding treatment strategies allows for more detailed evaluations about the treatments and avoid extraneous information from being documented.
Despite its purported advantages, there are challenges associated with the implementation of a clinical outcomes registry including costs (i.e., time, money, resources), coordination amongst multiple practitioners and clinic staff, and managing various cell mediated treatment strategies (5). The first challenge associated with the implementation of the clinical outcomes registry is the overall cost including time, money, and resources (20). The registry implementation needed to be efficient in order to minimize related costs for the facility. In addition, coordination amongst multiple practitioners and clinic staff may be a hindrance to the implementation and initial use of the clinical outcomes registry (21). Therefore, it was crucial to develop detailed guidelines and procedures to ensure staff using the registry daily are well versed in their roles. Finally, the management of various cell mediated therapies can be a major challenge for regenerative medicine facilities as the extremely detailed procedures and processes differ from most current registries that mainly focus on surgical intervention. The system had to provide all cell mediated therapies with a detailed framework to manage an array of factors, such as the frequency of treatment.
Methods
General overview
The primary purpose of the clinical outcomes registry was to objectively assess, analyze, and maximize patient-centered outcomes for surgical and non-surgical management of spine, knee, hip, shoulder and upper limb, and foot and ankle musculoskeletal disorders amongst the patient population. The secondary objective of the registry was to use clinical data to record the efficacy and safety of approved biological and interventional in order to improve their performance and direct future improvements in patient care. The registry was necessary to monitor both the natural history of orthopaedic pathologies occurring in the general population, as well as short-term and long-term patient outcomes associated with these pathologies and contemporary treatment options.
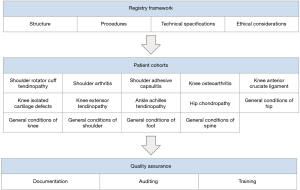
The registry was comprised of three sections: (I) registry framework; (II) the patient cohorts; and (III) quality assurance (Figure 2). In the first section, the general recruitment process of patients into the registry was documented and communicated using diagrams and step-by-step instructions. In the second section, the fourteen cohorts were document with their respective symptoms, treatments, and PROs to compare patients within each cohort. In the third section, the descriptions for how the data from the registry would be used to evaluate the quality of treatments, auditing treatment finances, or identifying issues to be solved through required and appropriate training.
Implementation process
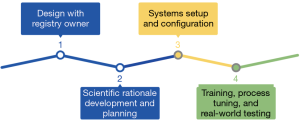
Implementing the clinical outcomes registry within an orthobiologics facility consisted of four steps (21) (Figure 3): (I) designing the structure of the registry with the intended owner; (II) utilizing previously published literature about regenerative medicine to develop scientific rationale for data collection points and methods for cell mediated treatment within the registry; (III) setting up and configuring the system within the facility; and (IV) training all medical staff that would be involved with patient flow throughout the registry process, making small adjustments based on the facility’s needs, and testing the registry with the population that will be participants in the future.
The first stage of building the registry consisted of a draft design. According to the aforementioned definition (5), a registry consists of “an organized system that uses observational study methods to collect uniform data… that serves a predetermined purpose,” therefore, registry designers worked extensively with the registry owner to establish the scope and scale of the registry (22). Within these interactions/conversations, preliminary work was conducted to connect the registry with the overall research program goals and design observational study methods that accurately allow for the purpose to be fulfilled through the uniform data.
Development of the scientific rationale for the registry required a solid understanding of what the registry owner (a clinician in this instance) wanted to know about their patients, treatments, and outcomes (21). The registry needed to have collection methods and data items that were applicable to the specific regenerative medicine treatments used. Therefore, registry information about these treatments was derived from the current literature about orthobiologics. Understanding that the registry structure is in a cohort format, each grouping had specific outcome measurements that were unique to those musculoskeletal disorders. Therefore, the recovery goals through various treatments were specific to each cohort. Additionally, literature relevant to each cohort was analyzed to determine the relevant diagnoses, the treatment pathways for the cohort, the most appropriate follow-up intervals, and which outcome measurements were to be used to establish treatment efficacy. Each cohort’s context within literature and plan for diagnosis, treatment, and analyses were thoroughly documented and made available to a broad range of registry stakeholders throughout the development period.
A number of standards operating procedures were necessary in order for the registry to maintain its integrity and to align with local regulatory and international best practice guidelines for human subjects research and good clinical practice. In designing the registry, informed consent, data protection, marketing practices, and the credentials of Investigators were emphasized. In designing the registry, informed consent was approached as an opportunity to communicate with patients about the nature of the registry, manage their expectations, their right to refuse or withdraw, and prepare them for further communication. Data use and protection was one area of particular concern for our patient population, and detailed procedures-mentioned in the Informed Consent-were developed in order to allay fears related to data security.
Coordination between the registry designers and the clinicians was crucial during the system setup and configuration step of the implementation. Identifiable data was kept electronically on a secure server and all identifying information such as name, date of birth, email, or phone was removed from any data prior to transfer of the data to sites. The physical terminal server was located at the facility and was backed up daily. Regular quality control audits were conducted to ensure data quality is at an adequate standard for clinical research. Registry level completeness audits were performed every 2 weeks until the benchmark 90% of potential patients was reached and performed monthly once above the benchmark (2).
As mentioned before, coordination amongst multiple practitioners and facility staff may be a hindrance to the implementation and initial use of the clinical outcomes registry. Detailed descriptions of each person’s role within the registry documentation process were provided in order to prevent important information from being neglected. Additionally, coordination between the registry support team and the local team proved difficult. Therefore, in-depth training and fine tuning for clinicians, nursing staff, clinical support, admin staff, and the registry support team was crucial for the success of the final implementation step. Online, in-person, and remote support was used to deliver ongoing training and process correction. Additionally, training from the registry support team was provided to ensure that faculty understood and were able to proficiently input data into the registry. Depending on the clinic, it’s facilities, staff size, etc., the specific roles of faculty can be adjusted and fine-tuned to promote a smoother process.
Finally, testing the efficacy of the registry itself within the new clinical environment was only possible through real-world testing. Once the training was completed and the registry fully created, then a small population of patients were added to the registry to analyze the process flow. Starting with a small, pilot group allowed for unforseen adjustments to be made without disrupting many patients. After the clinicians and registry support team were content with the overall registry and patient flow, the registry was fully implemented with all patients.
Results
The effect of the change in clinical practice was assessed through quality metrics associated with the registry itself, such as the accuracy of the data linked to supplementary data (e.g., clinic documentation). Once the registry completed its implementation phase and entered a pilot period to confirm data collection processes and user feedback. Initial findings indicated missing data in key areas that were rectified by refining data fields definition, placement within the operating system, linkage to external supporting documents and response options for users to select during data entry. Improvements to clinical practice were assessed through data quality assessment, patient outcomes relative to pre-specified treatment success criteria, as well as safety relative to external sources for benchmarking. In addition, the experience of users was assessed at the end of the initial pilot period (3 months) and changes to clinical practice over the course of the registry implementation have been noted in a documentation manual to provide context to data analysis in the future.
The key impacts of the registry implementation have been to (I) redefine criteria for treatment success and failure within the area of biologic treatments in musculoskeletal practice (5); (II) instigate between-practitioner discussion and documentation regarding standardising treatment pathways (11), clinical handover processes and shared decision-making with patients (7); and (III) act as a catalyst to target deficiencies in staff knowledge and skills in the areas of patient management and interaction (18), clinical documentation and administration processes (4).
Conclusions
The registry implementation established a series of processes within the facility, linked to the latest evidence, to identify patient outcomes with respect to treatment of musculoskeletal conditions. The success of introducing a registry in this environment depended on the organisation of the clinic and the leadership of key stakeholders within the process, both in the design and the practical translation of data collection processes to action. Standardisation of treatment pathways were required to anchor data collection processes and provide meaningful feedback on patient outcomes.
The change process was hampered by a lack of interoperability between electronic data systems, issues of standardisation of definitions leading to duplication of data collection between staff groups and the definition of treatment in the context of nonoperative management and biologic therapy in the context of following up patients at predefined time periods. Therefore, to combat these issues that emerged during implementation, communication needed to be formalized with all stakeholders earlier in the planning process to gather feedback on data collection logistics from the clinic staff as part of the design phase.
However, there are general constraints and limitations on the effectiveness of a practice registry. First, the limited access to the owner can prevent smooth communication between registry staff and support. Second, communication access depending on the location can inhibit the ease of communication between registry faculty and support. Third, the differing levels of knowledge and experience within the clinic environment incorporates multiple stakeholders into the patient flow through the registry which can prove a hindrance and limit the effectiveness of a practice registry.
Nevertheless, the implementation of a practice based patient registry enabled clinicians to not only keep track of their own performance, but to contribute to the evidence regarding emerging therapies in a systematic manner by monitoring patient outcomes. In addition, the introduction of a practice registry provided a platform for monitoring treatment safety and efficacy in the context of biologic therapies in musculoskeletal medicine and to contribute to ongoing discourse regarding best value treatments for a range of disabling conditions.
The aim of this didactic article was to describe the implementation of a clinical outcomes registry within a clinical setting for regenerative medicine. Through a detailed explanation of the implementation process, this registry style can be adapted to support regenerative medicine clinics worldwide. Although the registry in question only completed its pilot phase, adjustments are able to be made in order for the registry to serve its full purpose to address quality issues of feedback agility and specificity. Future work should examine the quality of the data held by the registry and begin to interpret the patient data captured during its pilot phase.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Murrell WD, Anz AW, Badsha H, et al. Regenerative treatments to enhance orthopedic surgical outcome. PM R 2015;7:S41-52. [Crossref] [PubMed]
- Gray JM, Abbasi K. How to get better value healthcare. J R Soc Med 2007;100:480. [Crossref]
- Abdulwahab TA, Murrell WD, Jenio FZ, et al. Complete Rotator Cuff Tear: An Evidence-Based Conservative Management Approach. Intech Open 2018. doi: [Crossref]
- Chiauzzi E, Rodarte C, DasMahapatra P. Patient-centered activity monitoring in the self-management of chronic health conditions. BMC Med 2015;13:77. [Crossref] [PubMed]
- Gliklich RE, Dreyer NA, Leavy MB. Registries for Evaluating Patient Outcomes: A User’s Guide. Agency for Healthcare Research and Quality (US), Rockville (MD), 2014.
- Centeno CJ, Al-Sayegh H, Freeman MD, et al. A multi-center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopaedic conditions. Int Orthop 2016;40:1755-65. [Crossref] [PubMed]
- Banerjee AK, Ingate S, Mayall S, et al. Patient Reported Outcomes (PRO) are Key to Post Launch Safety and Risk Management. Pharmacoepidemiol Drug Saf 2012;21:213-4.
- Forsberg HH, Nelson EC, Reid R, et al. Using patient-reported outcomes in routine practice: three novel use cases and implications. J Ambul Care Manage 2015;38:188-95. [Crossref] [PubMed]
- Jain NB, Kuye I, Higgins LD, et al. Surgeon volume is associated with cost and variation in surgical treatment of proximal humeral fractures. Clin Orthop Relat Res 2013;471:655-64. [Crossref] [PubMed]
- Makhni EC, Steinhaus ME, Swart E, et al. What Are the Strength of Recommendations and Methodologic Reporting in Health Economic Studies in Orthopaedic Surgery? Clin Orthop Relat Res 2015;473:3289-96. [Crossref] [PubMed]
- James BC, Savitz LA. How Intermountain trimmed health care costs through robust quality improvement efforts. Health Aff (Millwood) 2011;30:1185-91. [Crossref] [PubMed]
- Choinière M, Ware MA, Pagé MG, et al. Development and Implementation of a Registry of Patients Attending Multidisciplinary Pain Treatment Clinics: The Quebec Pain Registry. Pain Res Manag 2017;2017:8123812. [Crossref] [PubMed]
- Lecluse LLA, Naldi L, Stern RS, et al. National registries of systemic treatment for psoriasis and the European “Psonet” initiative. Dermatology 2009;218:347-56. [Crossref] [PubMed]
- Allen KD, Kasarskis EJ, Bedlack RS, et al. The National Registry of Veterans with amyotrophic lateral sclerosis. Neuroepidemiology 2008;30:180-90. [Crossref] [PubMed]
- Centeno CJ, Schultz JR, Cheever M, et al. Safety and complications reporting update on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell Res Ther 2011;6:368-78. [Crossref] [PubMed]
- Centeno CJ, Al-Sayegh H, Bashir J, et al. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. J Pain Res 2015;8:269-76. [PubMed]
- Nelson EC, Dixon-Woods M, Batalden PB, et al. Patient focused registries can improve health, care, and science. BMJ 2016;354:i3319. [Crossref] [PubMed]
- Wicks P, Hotopf M, Narayan VA. It’s a long shot, but it just might work! Perspectives on the future of medicine. BMC Med 2016;14:176. [Crossref] [PubMed]
- McLean L.. Clinical Trial Registries: An Industry Perspective. Drug Inf J 2010;44:279-87. [Crossref]
- Korir A, Gakunga R, Subramanian S, et al. Economic analysis of the Nairobi Cancer Registry: Implications for expanding and enhancing cancer registration in Kenya. Cancer Epidemiol 2016;45:S20-9. [Crossref] [PubMed]
- Latham T, Malomboza O, Nyirenda L, et al. Quality in practice: implementation of hospital guidelines for patient identification in Malawi. Int J Qual Health Care 2012;24:626-33. [Crossref] [PubMed]
- Hudelson P, Dominicé Dao M, Durieux-Paillard S.. Quality in practice: integrating routine collection of patient language data into hospital practice. Int J Qual Health Care 2013;25:437-42. [Crossref] [PubMed]