
Lactate and cancer: a “lactatic” perspective on spinal tumor metabolism (part 1)
Introduction to the two-part series
Surgical treatment of tumors about the spine remains a critical and often necessary component of caring for the patient with a spinal tumor. It is becoming increasingly common for patients with metastatic cancer to live for many years, often leading to the need for surgery to decompress and/or stabilize the spine (1). In 2005, Patchell et al. (2) published the landmark study demonstrating that radiation and surgery were more beneficial than radiation alone in patients with compressive, metastatic lesions of the spine. Though most patients with metastatic disease of the spine remain asymptomatic, prior population-based studies suggest that approximately 15% of patients will require surgical management, either for mechanical instability or epidural cord compression (3). Although less common than spinal metastases (4), primary tumors of the spine also commonly require complex operative management. For primary vertebral body malignancies, this involves an Enneking-appropriate resection (5,6), which often begets significant side effects (7) and is only justifiable given the significant survival benefits that it affords (8,9). Given the significant morbidity associated with these surgeries and with other current adjuvant therapies (e.g., radiation, chemotherapy), interest has grown in discovering other methods of targeting tumors of the spine. Recent research efforts have highlighted the tumor microenvironment, and specifically lactate metabolism, as central to tumorigenesis (10-15). In this two-part review, we detail the complicated history of research into metabolism as it pertains to both lactate and cancer and the tumor microenvironment (Part 1) before moving on to discuss specific mechanisms and reactions that may allow tumor-specific targeting (Part 2).
Introduction
In recent years there has been an increased focus on the metabolism of tumors (10,16). Specifically, there has been increased interest in the “Warburg effect,” whereby tumor cells are described as being glucose-avid and lactate-producing, despite normoxic conditions (17). While increased lactate production in the absence of hypoxia was originally described as paradoxical, it is now well-known that increased lactate production due to hypoxia is the exception rather than the rule (18,19). To this point, research on lactate metabolism in muscle over the past three decades has routinely demonstrated that increases in lactate concentration from exercise are rarely if ever caused by any oxygen limitation (11,15,20). Instead, circulating catecholamines bind β-adrenergic receptors on muscle cells, leading to the breakdown of muscle glycogen to lactate, which is then released down its concentration gradient into circulation, where it can circulate throughout the body. For example, during progressive, incremental exercise, catecholamines appear to play a significant role in the increase in [lactate] (21). Similarly, the increased [lactate] seen in many trauma patients, for many years thought to be due to “underresuscitation”, is also likely due in large part to the increased circulating catecholamines. This functions to mobilize otherwise inaccessible muscle glycogen, a well-preserved evolutionary response in times of emergency (recall muscle glycogen cannot leave a muscle cell as glucose) (21). It is now well-accepted that lactate circulates throughout the body and is used by almost all tissues as a fuel (15); remarkably, circulating lactate is the mechanism by which whole body metabolism is coordinated (18,19,22-24). Further, recent research has now detailed lactate’s integral role in angiogenesis through upregulation of hypoxia-inducible factor 1α (HIF-1α) and vascular endothelial growth factor (VEGF) (12). Finally, several studies have suggested that lactate plays a critical role in both tumor growth and progression to metastatic disease (25-30). Given its central role in metabolism, this is not overly surprising.
In light of these advances, lactate is now being considered as an integral component of both primary and metastatic cancer metabolism. New investigations in lactate homeostasis with a focus on identifying novel therapeutic targets are now underway. For example, Nijsten and van Dam (13) originally proposed that tumors might be targeted by lowering systemic [glucose] while simultaneously infusing lactate for healthy tissues to use as fuel [i.e., a “lactate-protected hypoglycemia” (LPH)] (10). However, others have shown that some cancer cells likely use lactate too, both as a fuel and/or angiogenic signal (12,31), possibly conflicting with the utility of a “lactate-protected hypoglycemia” as originally proposed (10). Thus, the purpose of this paper is to review the pertinent literature on lactate metabolism and tumor metabolism with the hope of (I) clarifying the roles of lactate in the tumor microenvironment, and (II) directing future research toward the development of new, targeted, and well-tolerated cancer therapies.
Lactate metabolism
Lactate, not ‘lactic acid,’ is formed in vivo (Figure 1), and remains >99% dissociated due to its pKa of ≈3.86 (11). Resting [lactate] in human blood is around 1.0mM and can increase to >15 mM after a short bout of maximal intensity effort of 60–90 s (32). In this review, the terminology “lactate” {and “[lactate]” for lactate concentration} will be used. Unless stated otherwise, this will refer to L(+)-lactate, the predominant form of lactate in the body (Note that the enantiomer D(−)-lactate can be found in vivo but is <0.2 mM and is not the product of glycolysis) (33).
It is also worth mentioning that “lactate flux” or “lactate clearance” are very different from “[lactate]”, despite the misuse by many authors [see Rothberg and Goodwin (34)]. Any given [lactate] is the net result of lactate production and lactate removal; both increasing production or decreasing removal can increase [lactate], and vice versa. Said a different way, the same [lactate] could be maintained in two separate cases, one with high lactate production (and high clearance), and the other with low lactate production (and low clearance). Mazzeo et al. (35) demonstrated thirty years ago that [lactate] was a very poor proxy for lactate clearance. Finally, there are differences in [lactate] between arterial and venous blood, but also between serum and whole blood. The latter is beyond the scope of this review (whole blood lactate is on average ≈0.70 of serum values due to Donnan equilibrium [see Goodwin et al. (32)]. The former is obvious, but should be reviewed: [lactate] of any venous blood draining a tissue bed is reflective of that local tissue, not the whole organism. Arterial [lactate], on the other hand, is the same throughout the body, as by definition no tissue exchange has taken place.
First discovered in sour milk by Swedish Apothecary and Chemist Carl Wilhelm Scheele in 1780, lactate was coined “Mjolksyra” or “acid of milk” (11). Scheele discovered many other compounds (e.g., tungsten, barium, chlorine, arsenic) and was the first person to discover oxygen, although he erroneously is often only given shared credit for it [see Ferguson et al. (11)]. It was not until 1807 that the existence of lactate in animals was discovered by Berzelius, who noted high levels of lactic acid in muscles of freshly slaughtered, “hunted stags” (36,37). The difference in chemical properties of animal lactate and the fermented milk lactate were soon described [which we of course now know was the difference between L(+)-lactate and D(−)-lactate]. For an excellent account of the history of early lactate discoveries, the interested reader is directed to Ferguson et al. (11).
Erroneous views on lactate and hypoxia
For our purposes, we revisit the early history of lactate research to lend context to the erroneous view that lactate is a “dead-end waste product” associated with illness, fatigue, and hypoxia (or hypoperfusion). These erroneous conclusions were repeated over and over, as scientists and physicians alike worked to fit new data into an incorrect and outdated paradigm. Lactate pioneer (and discoverer of the lactate shuttle) George Brooks called this one of the “great mistakes in the history of science” (38). After the discovery of elevated [lactate] in hunted stags by Berzelius, over 100 years of research followed which ultimately depicted lactate as a byproduct formed during conditions of muscular contractions, illness, or hypoxia (11,18). Further contributing to this was misinterpretation of the work of Louis Pasteur, who demonstrated that alcoholic fermentation by yeast could be inhibited by the addition of oxygen (39). Scientists drew false parallels between these findings and evidence demonstrating increased lactate production from glycogen in contracting muscle. In light of Pasteur’s work and other experiments demonstrating elevated lactate concentrations in oxygen-deprived muscle preparations, it was concluded that increased lactate production was likely a consequence of hypoxia. To this day data are still fit to this erroneous paradigm. For example, in the trauma setting, a persistent elevation in arterial [lactate] has been referred to as “occult hypoperfusion,” even in the absence of other signs of hypoxia/hypoperfusion [e.g., (40,41)]. We now know that any hypoxia-driven increase in [lactate] is the rare exception rather than the rule.
This concept slowly but definitively began to change in the late 1960’s when Wendell Stainbsy (42) at the University of Florida showed that the contracting, lactate-releasing dog muscle was not hypoxic. While his exact methods of these early experiments have been criticized, the concept of what he discovered ultimately changed our understanding of metabolism, which now includes our understanding of tumor metabolism. Later, in an attempt to experimentally make oxygen the rate-limiting factor, Richardson and colleagues (20) utilized a leg-kick model and measured intracellular PO2. Even with an isolated muscle group working to exhaustion, in humans breathing hypoxic gas (12% O2), they were unable to lower intracellular PO2 enough to limit oxidative phosphorylation. With this and other exercise studies designed to examine this concept [e.g., (43)], it has become increasingly clear that elevations in [lactate] during exercise are not due per se to an oxygen limitation.
In the trauma literature, a prolonged elevation in arterial [lactate] remains a poor prognostic indicator. For example, mortality is markedly elevated (18.8%) in trauma patients who present with an arterial [lactate] >4.0 mM. However, of that group, those with arterial [lactate] concentrations that dropped by >60% in the first 6 h had a dramatic reduction in mortality (7.5%), while those with <30% improvement at 6 h had a mortality of 28.1% (44). Similarly, poorer survival has been noted in trauma patients where arterial [lactate] was not reduced below 2.5 mM within 24 h (45). Though several critical thresholds have been published, in over 20+ years of study on critically ill and surgical trauma patients, the conclusions remain the same—a persistently elevated [lactate] confers a poor prognosis (41,46-48). Though the exact etiology has not been elucidated, this elevated mortality may stem from increased infection risk, as prior work has demonstrated a four-fold higher infection risk and seven-fold higher mortality among trauma patients with a persistently elevated arterial [lactate] (>2.5 mM at 12 h) as compared to matched controls (40).
Many providers discuss treating elevated lactate concentrations by administering more fluid, even in the absence of any markers supporting a hypoperfused state (e.g., hemodynamic instability, decreased urine output). This isolated elevated [lactate] has been called “occult hypoperfusion” by some in an effort to fit the clinical situation to their understanding of an elevated [lactate] (i.e., hypoperfusion or hypoxia). To defend this position some have implicated visceral organ hypoperfusion, but experimental data to support this are lacking. As one example, Tenhunen et al. (49) performed stepwise superior mesenteric artery (SMA) occlusion in pigs and arterial [lactate] was measured. Even with complete occlusion of the SMA there was no significant difference in arterial [lactate]. Others have proposed that microcirculation in the viscera drives this elevated [lactate]; however, when this is corrected there is no change in [lactate] (50). In fact, the catecholamine response to stress is now well-known to both drive increases in [lactate] in normoxic conditions, and greatly increase susceptibility to infection (51,52).
Lactate shuttle and catecholamine response
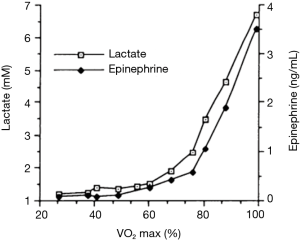
If not an oxygen or perfusion limitation, is it the catecholamine response that typically drives increases in [lactate], and might that lend insight into cancer metabolism? Although an exhaustive review of the exercise literature is well beyond the scope of this review, fundamental concepts lend insight. The lactate threshold is the work rate or VO2 beyond which [lactate] increases exponentially (32). Increases in [catecholamine] mirror the blood lactate kinetics and likely drive much of this response (21) (Figure 2). However, those changes in [lactate] are short-lived and arterial [lactate] returns to baseline within 2 h of exercise termination, in sharp contrast to the persistent elevation of [lactate] seen in trauma patients, which may persist for more than 24 h. Burn patients, in contrast, may have an elevated [lactate] for days to weeks, with a persistently elevated [catecholamine] that directly mirrors the lactate response (53).
Additionally, this elevated [lactate] in burn patients does not appear to be derived from the injured tissue. Gore et al. (54) examined this elevated [lactate] in patients 2 weeks after severe burns. They found that the lower extremities were the largest producers of lactate, regardless of the burn location. Further, administration of β-blockade greatly reduced this [lactate], consistent with the suspected catecholamine-dependent release of lactate from muscle glycogen. Daniel et al. (55,56) used a canine model to demonstrate dramatic reductions in muscle glycogen after induced shock. After shock states, animals had markedly elevated muscle tissue [lactate], which was slightly greater than the elevated arterial [lactate], which itself was greater than the other organs (i.e. there was a lactate gradient from reservoir to usage sites). Similarly, Irving (57) used a sheep model of hemorrhage shock to demonstrate that β-blockade completely attenuated the post-shock elevation in [lactate]. Finally, Liddell et al. (58) demonstrated catecholaminergic signaling to be both necessary and sufficient for the shock-driven elevation in [lactate]. In the animal shock model in dogs he was able to clearly and convincingly demonstrate that this rise in [lactate] diminished once catecholamines were depleted; upon exogenous addition of catecholamines the [lactate] again rose.
We have evolved such that situations of high stress (e.g., trauma, burn, hard exercise) invoke the release of catecholamines, which then bind muscle cells throughout the body. Because so much of human mass is muscle, it serves as a large reservoir of stored nutrients in the form of glycogen, which can quickly be broken down to lactate in response to catecholamine binding. Once formed, lactate can then diffuse out of the cell [mainly via monocarboxylate transporters (MCTs)] and circulate to the rest of the body, where it can be taken up and used as a fuel. It should be pointed out that this process requires relatively little immediate energy input. MCTs are diffusion-driven and thus lactate can move freely across these transporters down its concentration gradient (in or out of cells). Once lactate is intracellular, the lactate dehydrogenase (LDH) reaction is a rapid, near-equilibrium reaction (deltaG≈0); any additional lactate necessitates pyruvate formation, and vice versa (22). Note that the near-equilibrium state of the LDH reaction and its high activity in vivo dictate that it immediately equilibrates; it does not per se dictate what the actual concentration of lactate or pyruvate are at that equilibrium. In the case of LDH, the fastest enzyme in glycolysis, the equilibrium lies heavily toward lactate (59). This elegant way of coordinating whole body metabolism was first suspected when George Brooks proposed the lactate shuttle in the 1980s (23).
After Stainsby’s groundbreaking studies (42) showing that lactate production was not due to hypoxia, a series of experiments began involving isotopic tracers, muscle biopsies, lactate infusions, and determination of arteriovenous differences by Brooks and others that led to the formation of the cell-to-cell lactate shuttle hypothesis. Briefly, this posits that lactate, rather than being a dead-end metabolite, is a dynamic fuel that is circulated throughout the body and readily used as an oxidative fuel by almost all tissues of the body. This once hypothesis has now become a well-accepted theory (10,15) and is the lens through which current metabolic experiments should be viewed.
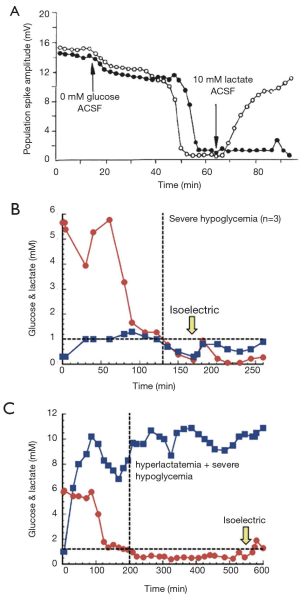
Data continue to accrue to this day that demonstrate the critical role lactate plays in whole body metabolism. Lactate flux, for example, often exceeds glucose flux (11,15,60,61). Further, numerous studies now support the use of lactate as a fuel by tissues throughout the body, including heart, muscle, kidney, and even the brain. Further review of specific lactate shuttles (e.g., the astrocyte-neuron lactate shuttle) is beyond the scope of this review [see (18)]. The importance of lactate in whole body metabolism is underscored by (I) experiments demonstrating tissues that prefer lactate over glucose, and (II) controlled experiments as well as case reports demonstrating that lactate can almost fully replace glucose as a fuel. For example, numerous studies exist demonstrating the ability of lactate to replace glucose as a fuel, perhaps best demonstrated by Avital Schurr’s work on recovering hippocampus slice function, first published in Science and shown here in Figure 3A (62). While clinical evidence is more difficult to interpret, the same principles appear valid. A case study from the Netherlands was reported (63) of a patient with profound, “nonsurvivable” hypoglycemia (0.7 mM) due to liver failure who was awake and alert, presumably due to his use of lactate as a fuel (he was found to be hyperlactatemic to 25 mM!). Newborns, who have immature livers, have an increased expression of lactate transporters and also may have [lactate] on the order of ≈6–9 mM, likely for this same reason (64,65). Finally, work from the Gladden lab at Auburn University reproduced this “lactate-protected hypoglycemia” (LPH) in a dog model of hypoglycemia. Dogs made extremely hypoglycemic (<1 mM) died within 30 min (Figure 3B) while dogs that were made extremely hypoglycemia but also treated with a lactate infusion to clamp arterial [lactate] at ≈9 mM lived for over 5 h (Figure 3C) (11).
Lactate and cancer
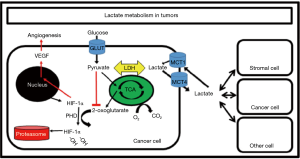
Evidence of tumors dates back to even ≈3,000 BC with descriptions of breast masses (66). However, it was the 1920’s that brought a plethora of experiments exploring tumors and more specifically, tumor metabolism. Tumors in solution were observed to acidify their environment with the addition of glucose, and an elevated [lactate] was measured (11,67). Warburg subsequently calculated lactate production in isolated rat hepatoma slice preparations and demonstrated production to be over 70 times that seen in normal tissue, independent of oxygen tension (68). Later the Cori’s (69) reported that venous blood from a sarcoma-bearing wing was higher in [lactate] {and lower in [glucose]} than the contralateral wing (without sarcoma). Warburg also used a rat model and found that the artery feeding the tumor was always higher in [glucose] and lower in [lactate] than the vein draining it (70). In total, this glucose-avid, lactate-producing behavior in normoxia was soon after dubbed “Warburg effect” (39).
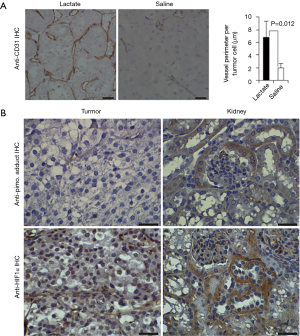
In the intervening 100 years since these discoveries from the Cori’s and Warburg, advances in both the study of metabolism as well as cancer have shed light on these early experiments. But with the dramatic advances made in cancer genetics in the late 20th century, the study of metabolism seemed to take a back seat. However, work done by Sonveaux et al. (71) demonstrated that there may be a lactate shuttle even within tumors, whereby cells at the hypoxic core produce lactate while those more peripheral may take up and oxidize that lactate as fuel. This led Gregg Semenza (the discoverer of HIF-1α) to take note and comment, “Was there any precedent that should have alerted us to the existence of this symbiotic relationship between aerobic and hypoxic cancer cells? Of course; the well-known recycling of lactate in exercising muscle.” (72). Since this, others have proposed and provided evidence that there is a “reverse Warburg” effects, where cancer cells are consumers of the lactate produced by noncancerous cells in the tumor microenvironment. At its most extreme, the Kevin B. Jones lab in Utah created a novel, genetically-engineered mouse model of Alveolar Soft Parts Sarcoma (ASPS) and incidentally discovered that tumors preferentially formed in areas of high [lactate]. On administration of daily lactate injections for 2 weeks, tumors became more vascular and demonstrated upregulation of proliferative markers despite normoxic conditions (Figure 4) (12).
Clinical prognosis
Clinically, changes in lactate metabolism have prognostic value for patients with multiple different primary cancers (73,74). A meta-analysis by Yao et al. (73), including over 1,800 patients with solid tumors, found that LDH5 overexpression was associated with a 60% overall higher risk of mortality. In a more recent series of 112 patients with triple-negative breast cancer, Huang et al. (75) demonstrated that higher expression levels of LDHA (LDH5) were not only associated with poorer overall survival, but also with more advanced TNM stage and a higher Ki67 proliferation index (75). Similar correlations between survival and LDHA levels have been reported in patients with pancreatic cancer (76), bladder cancer (77,78), cholangiocarcinoma (79), gastric cancer (80), renal cell carcinoma (81), and colorectal carcinoma (82). Chen et al. (83) reported LDH overexpression to be an independent negative predictor of postoperative survival in patients with hepatocellular carcinoma metastatic to the spine. Additionally, the authors found that the prognostic significance of LDH levels remained after application of current prognostic scoring systems, such as the Tokuhashi system. LDH levels also often correlate with tumor burden in human subjects (84).
Evidence to support the involvement of lactate metabolism in carcinogenesis has also been demonstrated in vitro and in vivo for lung (85), prostate (86), and breast cancers (87). Again, many of the key findings relate to changes in LDH expression, as it has been noted that LDH levels often track with overall survival, and often correlate with tumor burden. Knockdown of LDHA levels (88,89) and administration of LDH inhibitors hinder the growth of tumor cells in vitro (74,90,91).
Many tumor cells have been demonstrated to preferentially express LDHA (LDH5) (14), an isoform thought to shift the pyruvate-lactate equilibrium even more toward lactate (5). LDH is made up of four monomers [either “heart type” (H) or “muscle type” (M)] that are expressed from the Ldh-B (for “heart type”) and Ldh-A (for “muscle type”) genes and combine in various combinations (HHHH, HHHM, HHMM, HMMM, MMMM) to make five isozymes (LDH1 through LDH5). Often the LDH1 isoform is referred to as LDHB and the LDH5 isozyme is referred to as LDHA (11). While the LDH reaction is near-equilibrium and always lies heavily toward lactate (≈10:1 up to ≈100:1), the various LDH isozymes expressed are thought to influence how heavily toward lactate the reaction lies. For example, LDH-1 is thought to be more oxidative in nature while LDH-5 “more glycolytic” in nature. Circumstantial evidence has thus far supported this view (11), as oxidative tissues tend to express more LDH1 (and MCT1) than glycolytic tissues, which tend to express more LDH5 (and MCT4). However, it should be noted that the LDH reaction is a near-equilibrium reaction, and experts in the field of biochemistry have noted that the particular LDH isoform is likely less important than many have suggested (22,59). Please refer to Rogatzki et al. (22) for a thorough review of the issue. Nonetheless, lactate does appear to be implicated in aspects of tumorigenesis in many primary pathologies, and human studies evaluating clinically viable LDH inhibitors are currently pending (14,92).
Spinal tumor metabolism
Unlike tumors in limbs, tumors of the spine mandate narrow margins, the achievement of which may be improved through the use of neoadjuvant therapies to shrink the tumor. One promising avenue is the targeting of a cancer metabolic phenotype—a set of common metabolic features seen across multiple pathologies despite widely varying genetic profiles (93). However, it has become increasingly clear that deciphering whether a tumor or cancer type behaves in a “Warburg” or “reverse Warburg” manner (or somewhere in between) likely has to do with the specific tumor type, the local tumor conditions, as well as the systemic metabolic conditions {e.g., systemic [lactate]}. For example, Turner and Adamson (94) published a report examining the specific environmental factors that may influence the abnormal metabolism seen in astrocytomas, one of the more common intramedullary spinal tumors. The extracellular fluid throughout the central nervous system, for example, is kept closer to 2.0 to 2.5 mM by astrocytes that readily take up glucose and produce lactate for neurons to use as fuel (the astrocyte-neuron lactate shuttle) (18). This milieu of high [lactate] is thought to provide an environment suitable for tumor formation and progression, as will be discussed in the next sections. Outside of the spinal cord itself, other spinal tumors include primary tumors such as chondrosarcoma and osteosarcoma, as well as nonprimary tumors, such as metastatic disease, myeloma, and lymphoma. Obviously by no means an exhaustive list, a few representative studies from each demonstrates our relatively poor understanding of the tumor microenvironment as it applies to spinal tumors.
In vitro work with both chondrosarcoma and breast cancer cells has suggested them to have “Warburg” phenotypes. Hua et al. (95) used several chondrosarcoma cell lines and demonstrated that (I) chondrosarcoma cell lines had higher LDHA expression than wild type chondrocytes, (II) incremental increases in doxorubicin produced concomitant, stepwise increases in LDHA activity, and (III) LDHA inhibition restored doxorubicin-dependent cell killing, supporting the hypothesis that chondrosarcoma is an avid lactate producer, which likely contributes to its metastatic potential. Zhou et al. (96) discovered a similar phenomenon in breast cancer cells. Treatment of cells with paclitaxel led to an increase in LDHA expression in the paclitaxel-resistant cells. Subsequent knockdown of LDHA expression using small interfering RNA (siRNA) restored paclitaxel-mediated cell killing. Like the chondrosarcoma cells, these breast cancer cell lines (MDA-MB-231, BT474) were glucose-avid Warburg type cells. In both cases the production of lactate seemed to confer a survival advantage and possibly even impart some drug resistance. Though far from comprehensive, these studies demonstrate lactate production conferring a survival advantage to neoplastic cells.
To contrast these results, work with osteosarcoma cells, prostate cancer cells, and lung cancer cells appear to support a more reverse-Warburg type phenotype, where tumor cells consume lactate as a fuel. Bonuccelli et al. (97) utilized siRNA to known down MCT1s in human osteosarcoma cells while administering lactate. Usually ATP production increased when lactate was administered; with MCT knock-down, this response was blunted. Further, these authors demonstrated that cell migration significantly increased when lactate was administered, an effect which may underlie the observed connection of lactate to metastatic spread (25-30). Although it appears these cells were using lactate as both a fuel and to aid in mechanical migration, it must be pointed out that these cells were incubated with 10 mM [lactate], a level ≈10× in vivo resting conditions. Prior work from Pagliassotti and Donovan (98) has shown that above arterial [lactate] of ≈4 mM, even glycolytic, lactate-producing muscle will reverse and begin consuming lactate, complicating interpretation of many of these “reverse Warburg” studies. Further, osteosarcoma tumors are PET-positive tumors in vivo (although theoretically this could be due to either tumor cells or cancer associate fibroblasts taking up the 18F glucose analog).
Similarly, in some lung cancer and prostate cancer studies, lactate appears to be consumed by the tumor cells. In a comprehensive review of the literature on metabolism in prostate cancer, Pértega-Gomes and Baltazar (99) describe a mechanism whereby cancer associated fibroblasts upregulate lactate export via MCT4s and prostate cancer cells upregulate lactate import via MCT1s. In this manner prostate cancer cells may take up and use lactate as a fuel.
Work in lung cancer cells exhibits a similar phenotype, as detailed in a recent paper in Cell that spans experiments from mouse to human (85). Here, Faubert and colleagues at Texas Southwestern perform an elaborate series of studies demonstrating that non-small cell lung cancer cells likely use lactate as a fuel, similar to the model described for prostate cancer. Labeled 13C was used to show that lactate, not glucose, was responsible for the majority of tricarboxcylic acid (TCA) cycle carbon enrichment, a finding that has been corroborated in normal tissue and other tumor tissues (60), and serves to underscore the importance of lactate as the way whole body metabolism is coordinated. Weaknesses of this and other studies relying on tracers notwithstanding, these results and the already mentioned results from studies on the lactate-consuming ASPS tumors are intriguing; in many tumors lactate appears to play a significant role in tumorigenesis.
As one can now easily appreciate, the conditions in which these tumors are studied matters, particularly as it comes to explaining the observation of both lactate-producing and lactate-consuming phenotypes. As highlighted by Harjes (61), local tumor microenvironment is critical; intravascular injection of xenograft in the mouse produces tumors that are significantly more lactate-avid as compared to tumors produced through subcutaneous injection.
Similarly, both lymphomas and multiple myeloma have varying responses depending on other pertinent, local factors. Beloueche-Babari (100) used lymphoma cell lines with MCT1 inhibitor AZD3965, but cell death was not seen until this was used in concert with metformin, a mitochondrial inhibitor. This highlights the potential for synergistic combination therapies. Interestingly, multiple myeloma cells seem to both produce and consume lactate, depending on the cell line used. Sanchez et al. (101) used several different multiple myeloma cell lines to examine this. In the Warburg-type cells, administering dichloroacetate (DCA) to encourage conversion of pyruvate to acetyl-coA led to increased oxidative phosphorylation, but required a “second hit” agent to affect cell viability (in this case bortezomib, a proteasome inhibitor). Given these varying phenotypes, the critical role the tumor microenvironment plays, and the relatively untapped potential for targeted interventions, future investigation is warranted.
Conclusions and future directions
Tumors of the spine represent some of the most difficult tumors to treat, as surgery and other adjuvant therapies risk compromise of the spinal cord. In the search for new, novel therapies, lactate, once thought to be a metabolic waste product, has now emerged as a central player in tumorigenesis. To that end, many tumors are yet to be appropriately metabolically characterized, or have conflicting data depending on the model being used. Given the profound impact that the tumor microenvironment has on phenotype (and specifically whether a tumor cell is lactate-producing versus lactate-consuming), experimental models need to be optimized to address this (102), as current models do a poor job representing the in vivo human disease. Consequently, much of our current metabolic tumor knowledge should be carefully interpreted, and in the correct context.
For example, cell cultures do not replicate the spatial arrangement nor the dynamic chemical conditions present in vivo. In the study of tumors, both are critical components of the tumor microenvironment. To that end, animal models now more than ever provide the greatest promise for translating basic science discoveries into human cures. However, most mouse models lack spontaneously forming tumors, and instead may rely on an immunocompromised animal and/or an implanted cell line, both of which lead to an unnatural tumor and notably different tumor microenvironment (102,103). Genetically engineered mice can partially circumvent these problems and generate spontaneously-forming tumors, particularly if there is a known oncogene (12). Still, mice are too small to effectively alter or measure blood flow changes or metabolic kinetics (i.e., not enough blood volume for repeated draws or to pump-perfuse animal with its own blood). While one can infuse lactate to a desired concentration in dogs or humans and “clamp” it there (11,24,104), this is not possible in mice. Larger translational models represent an attractive method to bridge this gap. While animals such as goat, pig, rabbit, and other models exist, canine models are responsible for many of our current major advancements in medicine, including bone marrow transplantation protocols (105). In addition to the predictable physiological nature and proven track record of the canine in the lab, their blood volume allows for multiple and rapid serum sampling as well as continuous infusion of metabolic substrates of interest (e.g., lactate) (Figure 5). While many have dogs that live alongside them as companions, the United States alone euthanizes ≈3.4 million dogs and cats each year, mainly for population control (106). If even a few of those animals were used in humane, anesthetized, terminal experiments that shed light on cancer, we would undoubtedly be much closer to a cure for cancer than we currently are.

Dogs are also unique in that they have lived alongside humans for ≈15,000 to 100,000 years, sharing more “nature” (genetics) and “nurture” (environment) with humans than any other animal (107-109). Remarkably, they also form many spontaneous human cancers, like leukemia, lymphoma, osteosarcoma, soft tissue sarcomas, and melanoma, to name a few (110). In some cases dogs have a much higher incidence of specific rare cancers than humans. For example, dogs have ≈10 times higher incidence of osteosarcoma when compared to humans (103,110,111), representing a relatively untapped pool of a “pure” cancer model (spontaneously forming tumors). The National Cancer Institute (NCI) has taken note of this and formed the Comparative Oncology Program to study these animals (111). With the current shift back to an interest in tumor phenotype, rather than solely focusing on genotype, dog models represent perhaps our best chance of translating cancer research to cancer cure. Unfortunately, the shift of grant funding over the last several decades to mostly murine models has left few people trained to carry out experiments in dogs.
Finally, we conclude by noting that insight into lactate metabolism and its pro-oncologic effects might come from outside of the tumor or even lactate literature. For example, patients with von Gierke’s disease have an inability to form new glucose from gluconeogenesis or liberate liver glycogen due to glucose-6-phosphatase deficiency. These patients have hepatic lactate concentrations that can be dramatically elevated due to obligatory glycolysis to lactate (112). Remarkably, they also have an increased risk of hepatocellular carcinoma (113). Is this risk due to the elevated [lactate]? If so, perhaps efforts focused on mitigating the elevated [lactate] could improve the clinical course. Work in the last year from Cho et al. (114) demonstrates that an adeno-associated virus (AAV) can be utilized to restore glucose-6-phosphatase activity. Does this decrease cancer risk? On the other end of the metabolic spectrum is McArdle’s disease, in which a phosphorylase deficiency in the muscles causes an inability to breakdown muscle glycogen to lactate. Consequently, these patients have a well-documented inability to generate elevated lactate concentrations (115). Do they also have a lower risk of certain cancers than those without the disease? These studies are yet to be done, but might provide valuable insight.
Acknowledgments
None.
Footnote
Conflicts of Interest: ML Goodwin: Consultant for ROM3, Augmedics; DM Sciubba: Consultant for Orthofix, Globus, K2M, Medtronic, Stryker, Baxter. The other authors have no conflicts of interest to declare.
References
- Rose PS, Buchowski JM. Review Metastatic disease in the thoracic and lumbar spine: evaluation and management. J Am Acad Orthop Surg 2011;19:37-48. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet 2005;366:643-8. [Crossref] [PubMed]
- Sohn S, Kim J, Chung CK, et al. A nationwide epidemiological study of newly diagnosed spine metastasis in the adult Korean population. Spine J 2016;16:937-45. [Crossref] [PubMed]
- Sohn S, Kim J, Chung CK, et al. A nation-wide epidemiological study of newly diagnosed primary spine tumor in the adult Korean population, 2009-2011. J Korean Neurosurg Soc 2017;60:195-204. [Crossref] [PubMed]
- Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res 1980.106-20. [PubMed]
- Enneking WF. A System of Staging Musculoskeletal Neoplasms. Clin Orthop Relat Res 1986.9-24. [PubMed]
- Chi JH, Sciubba DM, Rhines LD, et al. Surgery for Primary Vertebral Tumors: En Bloc versus Intralesional Resection. Neurosurg Clin N Am 2008;19:111-7. [Crossref] [PubMed]
- Dekutoski MB, Clarke MJ, Rose P, et al. Osteosarcoma of the spine: prognostic variables for local recurrence and overall survival, a multicenter ambispective study. J Neurosurg Spine 2016;25:59-68. [Crossref] [PubMed]
- Schwab J, Gasbarrini A, Bandiera S. Osteosarcoma of the mobile spine. Spine (Phila Pa 1976) 2012;37:E381-6. [Crossref] [PubMed]
- Goodwin ML, Gladden LB, Nijsten MWN, et al. Lactate and Cancer: Revisiting the Warburg Effect in an Era of Lactate Shuttling. Front Nutr 2015;1:27. [Crossref] [PubMed]
- Ferguson BS, Rogatzki MJ, Goodwin ML, et al. Lactate metabolism: historical context, prior misinterpretations, and current understanding. Eur J Appl Physiol 2018;118:691-728. [Crossref] [PubMed]
- Goodwin ML, Jin H, Straessler K, et al. Modeling Alveolar Soft Part Sarcomagenesis in the Mouse: A Role for Lactate in the Tumor Microenvironment. Cancer Cell 2014;26:851-62. [Crossref] [PubMed]
- Nijsten MWN, van Dam GM. Hypothesis: Using the Warburg effect against cancer by reducing glucose and providing lactate. Med Hypotheses 2009;73:48-51. [Crossref] [PubMed]
- San-Millán I, Brooks GA. Reexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis 2017;38:119-33. [PubMed]
- Brooks GA. The Science and Translation of Lactate Shuttle Theory. Cell Metab 2018;27:757-85. [Crossref] [PubMed]
- Potter M, Newport E, Morten KJ. The Warburg effect: 80 years on. Biochem Soc Trans 2016;44:1499-505. [Crossref] [PubMed]
- Warburg O. On the Origin of Cancer Cells. Science 1956;123:309-14. [Crossref] [PubMed]
- Gladden LB. Lactate metabolism: A new paradigm for the third millennium. J Physiol 2004;558:5-30. [Crossref] [PubMed]
- Gladden LB. A. “lactatic” perspective on metabolism. Med Sci Sports Exerc 2008;40:477-85. [Crossref] [PubMed]
- Richardson RS, Noyszewski EA, Kendrick KF, et al. Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J Clin Invest 1995;96:1916-26. [Crossref] [PubMed]
- Garcia-Alvarez M, Marik P, Bellomo R. Stress hyperlactataemia: Present understanding and controversy. Lancet Diabetes Endocrinol 2014;2:339-47. [Crossref] [PubMed]
- Rogatzki MJ, Ferguson BS, Goodwin ML, et al. Lactate is always the end product of glycolysis. Front Neurosci 2015;9:22-7. [Crossref] [PubMed]
- Brooks GA. Lactate shuttles in Nature. Biochem Soc Trans 2002;30:258-64. [Crossref] [PubMed]
- Miller BF, Fattor JA, Jacobs KA, et al. Lactate and glucose interactions during rest and exercise in men: Effect of exogenous lactate infusion. J Physiol 2002;544:963-75. [Crossref] [PubMed]
- Schwickert G, Walenta S, Sundfør K, et al. Correlation of high lactate levels in human cervical cancer with incidence of metastasis. Cancer Res 1995;55:4757-9. [PubMed]
- Walenta S, Salameh A, Lyng H, et al. Correlation of high lactate levels in head and neck tumors with incidence of metastasis. Am J Pathol 1997;150:409-15. [PubMed]
- Walenta S, Wetterling M, Lehrke M, et al. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res 2000;60:916-21. [PubMed]
- Walenta S, Chau TV, Schroeder T, et al. Metabolic classification of human rectal adenocarcinomas: a novel guideline for clinical oncologists? J Cancer Res Clin Oncol 2003;129:321-6. [Crossref] [PubMed]
- Brizel DM, Schroeder T, Scher RL, et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys 2001;51:349-53. [Crossref] [PubMed]
- Hur H, Xuan Y, Kim YB, et al. Expression of pyruvate dehydrogenase kinase-1 in gastric cancer as a potential therapeutic target. Int J Oncol 2013;42:44-54. [Crossref] [PubMed]
- Sotgia F, Martinez-Outschoorn UE, Lisanti MP. The reverse Warburg effect in osteosarcoma. Oncotarget 2014;5:7982-3. [Crossref] [PubMed]
- Goodwin ML, Harris JE, Hernández A, et al. Blood Lactate Measurements and Analysis during Exercise A Guide for Clinicians. J Diabetes Sci Technol 2007;1:558-69. [Crossref] [PubMed]
- Ewaschuk JB, Naylor JM, Zello GA. D-Lactate in Human and Ruminant Metabolism. J Nutr 2005;135:1619-25. [Crossref] [PubMed]
- Goodwin ML, Rothberg DL. Lactate metabolism in trauma. J Trauma Acute Care Surg 2014;77:182-3. [Crossref] [PubMed]
- Mazzeo RS, Marshall P. Influence threshold of plasma catecholamines during graded exercise. J Appl Physiol (1985) 1989;67:1319-22. [PubMed]
- Gladden LB. 200th Anniversary of lactate research in muscle. Exerc Sport Sci Rev 2008;36:109-15. [Crossref] [PubMed]
- Jons Jacob Berzelius. (1779-1848). JAMA 1965;193:153-4. [Crossref] [PubMed]
- Kolata G. Lactic Acid is Not Muscle’s Foe, It’s Fuel. New York Times, 2006.
- Racker E. History of the Pasteur Effect and its pathobiology. Mol Cell Biochem 1974;5:17-23. [Crossref] [PubMed]
- Claridge JA, Crabtree TD, Pelletier SJ, et al. Persistent Occult Hypoperfusion Is Associated with a Significant Increase in Infection Rate and Mortality in Major Trauma Patients. J Trauma 2000;48:8. [Crossref] [PubMed]
- Crowl AC, Young JS, Kahler DM, et al. Occult hypoperfusion is associated with increased morbidity in patients undergoing early femur fracture fixation. J Trauma 2000;48:260-7. [Crossref] [PubMed]
- Jöbsis FF, Stainsby WN. Oxidation of NADH during contractions of circulated mammalian skeletal muscle. Respir Physiol 1968;4:292-300. [Crossref] [PubMed]
- Connett RJ, Gayeski TE, Honig CR. Lactate accumulation in fully aerobic, working, dog gracilis muscle. Am J Physiol 1984;246:H120-8. [PubMed]
- Odom SR, Howell MD, Silva GS, et al. Lactate clearance as a predictor of mortality in trauma patients. J Trauma Acute Care Surg 2013;74:999-1004. [Crossref] [PubMed]
- Blow O, Magliore L, Claridge JA, et al. The golden hour and the silver day: detection and correction of occult hypoperfusion within 24 hours improves outcome from major trauma. J Trauma 1999;47:964-9. [Crossref] [PubMed]
- Grey B, Rodseth RN, Muckart DJ. Early fracture stabilisation in the presence of subclinical hypoperfusion. Injury 2013;44:217-20. [Crossref] [PubMed]
- Venkatesan M, Smith RP, Balasubramanian S, et al. Serum lactate as a marker of mortality in patients with hip fracture: A prospective study. Injury 2015;46:2201-5. [Crossref] [PubMed]
- Richards JE, Matuszewski PE, Griffin SM, et al. The role of elevated lactate as a risk factor for pulmonary morbidity after early fixation of femoral shaft fractures. J Orthop Trauma 2016;30:312-8. [Crossref] [PubMed]
- Tenhunen JJ, Jakob SM, Takala JA. Gut luminal lactate release during gradual intestinal ischemia. Intensive Care Med 2001;27:1916-22. [Crossref] [PubMed]
- Puskarich MA, Shapiro NI, Massey MJ, et al. Lactate Clearance in Septic Shock is Not a Surrogate for Improved Microcirculatory Flow. Acad Emerg Med 2016;23:690-3. [Crossref] [PubMed]
- Freestone PPE, Sandrini SM, Haigh RD, et al. Microbial endocrinology: how stress influences susceptibility to infection. Trends Microbiol 2008;16:55-64. [Crossref] [PubMed]
- Garcia-Alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. Crit Care 2014;18:503-11. [Crossref] [PubMed]
- Cochran A, Edelman LS, Saffle JR, et al. The relationship of serum lactate and base deficit in burn patients to mortality. J Burn Care Res 2007;28:231-40. [Crossref] [PubMed]
- Gore DC, Honeycutt D, Jahoor F, et al. Propranolol diminishes extremity blood flow in burned patients. Ann Surg 1991;213:568-73; discussion 573-4. [Crossref] [PubMed]
- Daniel AM, Shizgal HM, MacLean LD. Endogenous fuels in experimental shock. Surgical Forum 1976;27:32-33. [PubMed]
- Daniel AM, Shizgal HM, MacLean LD. The anatomic and metabolic source of lactate in shock. Surgery, gynecology, and obstetrics. Surg Gynecol Obstet 1978;147:697-700. [PubMed]
- Irving MH. The sympatho-adrenal factor in haemorrhagic shock. Ann R Coll Surg Engl 1968;42:367-86. [PubMed]
- Liddell MJ, Daniel AM, MacLean LD, et al. The role of stress hormones in the catabolic metabolism of shock. Surgery, gynecology, and obstetrics. Surg Gynecol Obstet 1979;149:822-30. [PubMed]
- Bak LK, Schousboe A. Misconceptions regarding basic thermodynamics and enzyme kinetics have led to erroneous conclusions regarding the metabolic importance of lactate dehydrogenase isoenzyme expression. J Neurosci Res 2017;95:2098-102. [Crossref] [PubMed]
- Hui S, Ghergurovich JM, Morscher RJ, et al. Glucose feeds the TCA cycle via circulating lactate. Nature 2017;551:115-8. [Crossref] [PubMed]
- Harjes U. Metabolism: More lactate, please. Nat Rev Cancer 2017;17:707. [Crossref] [PubMed]
- Schurr A, West CA, Rigor BM. Lactate-supported synaptic function in the rat hippocampal slice preparation. Science 1988;240:1326-8. [Crossref] [PubMed]
- Oldenbeuving G, Mcdonald JR, Goodwin ML, et al. A patient with acute liver failure and extreme hypoglycaemia with lactic acidosis who was not in coma: Causes and consequences of Lactate-protected hypoglycaemia. Anaesth Intensive Care 2014;42:507-11. [Crossref] [PubMed]
- Rinholm JE, Hamilton NB, Kessaris N, et al. Regulation of oligodendrocyte development and myelination by glucose and lactate. J Neurosci 2011;31:538-48. [Crossref] [PubMed]
- Sánchez-Abarca LI, Tabernero A, Medina JM. Oligodendrocytes use lactate as a source of energy and as a precursor of lipids. Glia 2001;36:321-9. [Crossref] [PubMed]
- Mukherjee S. The emperor of all maladies: a biography of cancer. New York: Scribner, 2010.
- Warburg O, Minami S. No Versuche an überlebendem carcinomgewebe. Klin Wochenschr. Klin Wochenschr 1923;2:776-7. [Crossref]
- Otto AM. Warburg effect(s)—a biographical sketch of Otto Warburg and his impacts on tumor metabolism. Cancer Metab 2016;4:5. [Crossref] [PubMed]
- Cori CF, Cori GT. The carbohydrate metabolism of tumors. II. Changes in the sugar, lactic acid, and co-combining power of blood passing through a tumor. J Biol Chem 1925;65:397-405.
- Warburg O, Wind F, Negelein E. The metabolism of tumours in the body. J Gen Physiol 1927;8:519-30. [Crossref] [PubMed]
- Sonveaux P, Copetti T, De Saedeleer CJ, et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS One 2012;7:e33418. [Crossref] [PubMed]
- Semenza GL. Tumor metabolism: cancer cells give and take lactate. J Clin Invest 2008;118:3835-7. [PubMed]
- Yao Y, Wang H, Li B. LDH5 overexpression is associated with poor survival in patients with solid tumors: a meta-analysis. Tumour Biol 2014;35:6973-81. [Crossref] [PubMed]
- Liu X, Yang Z, Chen Z, et al. Effects of the suppression of lactate dehydrogenase A on the growth and invasion of human gastric cancer cells. Oncol Rep 2015;33:157-62. [Crossref] [PubMed]
- Huang X, Li X, Xie X, et al. High expressions of LDHA and AMPK as prognostic biomarkers for breast cancer. Breast 2016;30:39-46. [Crossref] [PubMed]
- Mohammad GH, Olde Damink SWM, Malago M, et al. Pyruvate Kinase M2 and Lactate Dehydrogenase A Are Overexpressed in Pancreatic Cancer and Correlate with Poor Outcome. PLoS One 2016;11:e0151635. [Crossref] [PubMed]
- Koukourakis MI, Kakouratos C, Kalamida D, et al. Hypoxia-inducible proteins HIF1α and lactate dehydrogenase LDH5, key markers of anaerobic metabolism, relate with stem cell markers and poor post-radiotherapy outcome in bladder cancer. Int J Radiat Biol 2016;92:353-63. [Crossref] [PubMed]
- Jiang F, Ma S, Xue Y, et al. LDH-A promotes malignant progression via activation of epithelial-to-mesenchymal transition and conferring stemness in muscle-invasive bladder cancer. Biochem Biophys Res Commun 2016;469:985-92. [Crossref] [PubMed]
- Thonsri U, Seubwai W, Waraasawapati S, et al. Overexpression of lactate dehydrogenase A in cholangiocarcinoma is correlated with poor prognosis. Histol Histopathol 2017;32:503-10. [PubMed]
- Sun X, Sun Z, Zhu Z, et al. Expression of SIP1 is strongly correlated with LDHA and shows a significantly poor outcome in gastric cancer. Tumour Biol 2015;36:7521-30. [Crossref] [PubMed]
- Girgis H, Masui O, White NM, et al. Lactate dehydrogenase A is a potential prognostic marker in clear cell renal cell carcinoma. Mol Cancer 2014;13:101. [Crossref] [PubMed]
- Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Prognostic and predictive role of lactate dehydrogenase 5 expression in colorectal cancer patients treated with PTK787/ZK 222584 (vatalanib) antiangiogenic therapy. Clin Cancer Res 2011;17:4892-900. [Crossref] [PubMed]
- Chen H, Xiao J, Yang X, et al. Preoperative scoring systems and prognostic factors for patients with spinal metastases from hepatocellular carcinoma. Spine (Phila Pa 1976) 2010;35:E1339. [Crossref] [PubMed]
- Milanovic N, Matkovic S, Ristic D, et al. Significance of tumor burden, vascular endothelial growth factor, lactate dehydrogenase and beta-2 microglobulin serum levels in advanced diffuse large B cell lymphoma. J Buon 2012;17:497-501. [PubMed]
- Faubert B, Li KY, Cai L, et al. Lactate Metabolism in Human Lung Tumors. Cell 2017;171:358-371.e9. [Crossref] [PubMed]
- Nenu I, Gafencu GA, Popescu T, et al. Lactate - A new frontier in the immunology and therapy of prostate cancer. J Cancer Res Ther 2017;13:406-11. [PubMed]
- Jia Z, Zhang J, Wang Z, et al. An explorative analysis of the prognostic value of lactate dehydrogenase for survival and the chemotherapeutic response in patients with advanced triple-negative breast cancer. Oncotarget 2018;9:10714-22. [Crossref] [PubMed]
- Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 2006;9:425-34. [Crossref] [PubMed]
- Vander Heiden MG. Targeting cancer metabolism: A therapeutic window opens. Nat Rev Drug Discov 2011;10:671-84. [Crossref] [PubMed]
- Fiume L, Manerba M, Vettraino M, et al. Impairment of aerobic glycolysis by inhibitors of lactic dehydrogenase hinders the growth of human hepatocellular carcinoma cell lines. Pharmacology 2010;86:157-62. [Crossref] [PubMed]
- Le A, Cooper CR, Gouw AM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci 2010;107:2037-42. [Crossref] [PubMed]
- Akhenblit PJ, Pagel MD. Recent Advances in Targeting Tumor Energy Metabolism with Tumor Acidosis as a Biomarker of Drug Efficacy. J Cancer Sci Ther 2016;8:20-9. [Crossref] [PubMed]
- Gladden LB, Goodwin ML, McDonald JR, et al. Fuel for cancer cells? Cell Cycle 2011;10:2421-2. [Crossref] [PubMed]
- Turner DA, Adamson DC. Neuronal-astrocyte metabolic interactions: Understanding the transition into abnormal astrocytoma metabolism. J Neuropathol Exp Neurol 2011;70:167-76. [Crossref] [PubMed]
- Hua G, Liu Y, Li X, et al. Targeting glucose metabolism in chondrosarcoma cells enhances the sensitivity to doxorubicin through the inhibition of lactate dehydrogenase-A. Oncol Rep 2014;31:2727-34. [Crossref] [PubMed]
- Zhou M, Zhao Y, Ding Y, et al. Warburg effect in chemosensitivity: targeting lactate dehydrogenase-A re-sensitizes taxol-resistant cancer cells to taxol. Mol Cancer 2010;9:33. [Crossref] [PubMed]
- Bonuccelli G, Avnet S, Grisendi G, et al. Role of mesenchymal stem cells in osteosarcoma and metabolic reprogramming of tumor cells. Oncotarget 2014;5:7575-88. [Crossref] [PubMed]
- Pagliassotti MJ, Donovan C. Role of cell type in net lactate removal by skeletal muscle. Am J Physiol 1990;258:E635-42. [PubMed]
- Pértega-Gomes N, Baltazar F. Lactate transporters in the context of prostate cancer metabolism: What do we know? Int J Mol Sci 2014;15:18333-48. [Crossref] [PubMed]
- Beloueche-Babari M, Wantuch S, Galobart TC, et al. MCT1 inhibitor AZD3965 increases mitochondrial metabolism, facilitating combination therapy and noninvasive magnetic resonance spectroscopy. Cancer Res 2017;77:5913-24. [Crossref] [PubMed]
- Sanchez WY, McGee SL, Connor T, et al. Dichloroacetate inhibits aerobic glycolysis in multiple myeloma cells and increases sensitivity to bortezomib. Br J Cancer 2013;108:1624-33. [Crossref] [PubMed]
- Muir A, Danai LV, Vander Heiden MG. Microenvironmental regulation of cancer cell metabolism: implications for experimental design and translational studies. Dis Model Mech 2018;11:dmm035758. [Crossref] [PubMed]
- Overgaard NH, Fan TM, Schachtschneider KM, et al. Of Mice, Dogs, Pigs, and Men: Choosing the Appropriate Model for Immuno-Oncology Research. ILAR J 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Fattor JA, Miller BF, Jacobs KA, et al. Catecholamine response is attenuated during moderate-intensity exercise in response to the “lactate clamp”. Am J Physiol Endocrinol Metab 2005;288:E143-7. [Crossref] [PubMed]
- Monroy RL. Improved survival of dogs exposed to fission neutron irradiation and transplanted with DLA identical bone marrow. Bone Marrow Transplant 1987;2:375-84. [PubMed]
- The Humane Society of the United States. Available online: https://www.humanesociety.org/, accessed Dec. 16, 2018.
- Kirkness EF, Bafna V, Halpern AL, et al. The Dog Genome: Survey Sequencing and Comparative Analysis. Science 2003;301:1898-903. [Crossref] [PubMed]
- Lindblad-Toh K, Wade CM, Mikkelsen TS, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 2005;438:803-19. [Crossref] [PubMed]
- Gardner HL, Fenger JM, London CA. Dogs as a Model for Cancer. Annu Rev Anim Biosci 2016;4:199-222. [Crossref] [PubMed]
- Sultan F, Ganaie BA. Comparative oncology: Integrating human and veterinary medicine. Open Vet J 2018;8:25-34. [Crossref] [PubMed]
- Gordon I, Paoloni M, Mazcko C, et al. The comparative oncology trials consortium: Using spontaneously occurring cancers in dogs to inform the cancer drug development pathway. PLoS Med 2009;6:e1000161. [Crossref] [PubMed]
- Oster Y, Wexler ID, Heyman SN, et al. Recoverable, Record-High Lactic Acidosis in a Patient with Glycogen Storage Disease Type 1: A Mixed Type A and Type B Lactate Disorder. Case Rep Med 2016;2016:4362743. [Crossref] [PubMed]
- Wang DQ, Fiske LM, Carreras CT, et al. Natural history of hepatocellular adenoma formation in glycogen storage disease type 1. J Pediatr 2011;159:442-6. [Crossref] [PubMed]
- Cho JH, Kim GY, Mansfield BC, et al. Hepatic glucose-6-phosphatase-α deficiency leads to metabolic reprogramming in glycogen storage disease type Ia. Biochem Biophys Res Commun 2018;498:925-31. [Crossref] [PubMed]
- Delaney NF, Sharma R, Tadvalkar L, et al. Metabolic profiles of exercise in patients with McArdle disease or mitochondrial myopathy. Proc Natl Acad Sci 2017;114:8402-7. [Crossref] [PubMed]


