
Transplantation of dental tissue-derived mesenchymal stem cells ameliorates nephritis in lupus mice
Introduction
Systemic lupus erythematosus (SLE) is a typically fatal autoimmune disease associated with multiple organ involvement including lupus nephritis, a serious complication with more than 50% occurrence in SLE patients (1,2). Due to various immunological, genetic and environmental factors, the pathogenesis of SLE is complicated and has not been fully elucidated. The most prominent feature of SLE is the production of antibodies that recognize nuclear elements and another self-antigen. These autoantibodies form immune complex and deposit in the kidney, which subsequently lead to lupus nephritis. Conventional immunosuppressive therapy is effective for most SLE cases, however, there are still numbers of SLE patients consistently suffering the active disease with visceral organ involvement (3). Thus, identifying novel therapeutic strategies is required for SLE treatment.
Mesenchymal stem cells (MSCs) are fibroblast-like multipotent cells that derived from a variety of tissues such as bone marrow (BM), umbilical cords (UC), adipose tissue (AD), placenta and dental pulp. They can regulate various immune cells, as T and B lymphocytes, DC and NK cells, as well as complement system (4). During the past 10 years, considerable experimental and clinical evidences have proved that human umbilical cord mesenchymal stem cells (UC-MSCs) were powerful in reducing autoantibody levels and SLE disease activity (5). Except for UC-MSCs, BM-MSCs, human embryonic stem cell (hESC)-derived MSCs, AD-MSCs and stem cells from human exfoliated deciduous teeth (SHED) are capable to effectively reverse SLE-associated disorders in murine model of lupus nephritis (6-9).
Human dental pulp stem cells (DPSCs) and periodontal ligament stem cells (PDLSCs) were isolated from adult dental pulp and periodontal ligament tissue respectively (10,11). Though both of them originate from neural crest and share similar surface markers, differentiation potential and immunosuppressive properties, they are considered distinct from each other. It was reported that DPSCs and PDLSCs reacted differently to osteogenic induction in vitro (10,11). Thus, whether they have different immunomodulatory properties is still unknown. The current study was conducted to determine the therapeutic effects of dental tissue-derived MSCs and elucidate the underlying mechanism.
Methods
Animal
B6.MRL-Faslpr/J (B6/lpr) mice (female, 26-week-old, n=40) were purchased from Laboratory Animal Center, Academy of Military Medical Sciences (Beijing, China) and kept in specific-pathogen-free (SPF) conditions in the animal center of Drum Tower Hospital. All the animal experiments were performed under protocols approved by the Ethics Committee for Animal Research in the Affiliated Drum Tower Hospital of Nanjing University Medical School.
MSCs
UC-MSCs were prepared as described previously (12), and cultured in DMEM/F12 supplemented with 10% FBS, 100 U/mL penicillin, 100 µg/mL streptomycin, and 2 mmol/L glutamine. DPSCs and PDLSCs, provided by Nanjing Taisheng Biotechnology Co., Ltd, were cultured in α-MEM supplemented with 10% FBS. All the cells were maintained in humidified atmosphere with 5% CO2 at 37 °C.
To examine the phenotype of MSCs, cells (1×105/100 µL) at passage 5 (P5) were prepared as single cell suspension by trypsin/EDTA digestion and re-suspended in RPMI1640 containing 1% FBS (Bioind, Israel) and incubated with indicated antibodies (5 µg/mL) for 30 min on ice. Antibodies reactive to CD14, CD34, CD44, CD45, CD73, CD79, CD90, CD105, CD106 and HLA-DR (eBioscience), or isotype control immunoglobulin (eBioscience) were used. After washing with phosphate buffered saline (PBS), the cells were acquired by a flow cytometer (Calibur, BD Biosciences, CA, USA). Fluorescence-activated cell sorting (FACS) data were analyzed with FlowJo software (Tree Star, USA).
For adipogenic induction, MSCs were cultured in DMEM/F12 medium with 10% FBS, and adipogenic supplements (10 µg/mL insulin, 60 µmol/L indomethacin, 500 nmol/L hydrocortisone, 500 nmol/L hydrocortisone (Sigma-Aldrich)). After 3 weeks, the cultured cells were stained with Oil Red-O (Sigma Aldrich).
For osteogenic induction, MSCs were cultured in DMEM/F12 medium with 10% FBS, and osteogenic supplements (10 nmol/L dexamethasone, 100 µmol/L L-ascorbic acid 2-phosphate, 2 mmol/L β-glycerophosphate (Sigma-Aldrich)). After 4 weeks of induction, the cultures were stained with alizarin red for mineralized nodule formation.
T cell proliferation assay
5×105 peripheral blood mononuclear cells (PBMC) from health donors were labeled with 5 µmol/L carboxyflurescein diacetate succinimidyl ester (CFSE) (eBioscience) and stimulated with 2 µg/mL anti-CD3 (OKT3)/CD28 antibodies in the presence or absence of 1×105 MSCs. Cells were cultured for 4 days at 37 °C in a humidified atmosphere with 5% CO2. PBMCs were then collected, and cell proliferation was analyzed by flow cytometry (Calibur, BD Bioscience).
Intravenous transplantation of MSCs
UC-MSCs, DPSCs and PDLSCs were collected and washed with PBS three times. Cells were resuspended in PBS and intravenously infused at 2×105 per 10 g body weight into 28-week-old B6/lpr mice. Age-matched B6/lpr mice receiving PBS were used as controls, 24-h proteinuria were measured every 3 weeks by Coomassie Brilliant Blue. All mice were sacrificed at the age of 38-week to evaluate the therapeutic effects of MSCs transplantation.
Pathology assessment of kidneys
Kidneys were collected when the mice were sacrificed at 10 weeks after MSCs treatment. One kidney was fixed with 4% paraformaldehyde in PBS, then embedded in paraffin and sectioned (4 µm). The sections were stained with hematoxylin and eosin (H&E), periodic acid-Schiff (PAS) and Masson trichrome (MASSON). The slides were examined in a blinded fashion and graded for glomerular pathology and perivascular cell accumulation according to the grading scheme, the method reported by Chan and Kikawada (13,14).
Immunofluorescence
One kidney was snap-frozen in liquid nitrogen and placed in OCT medium, and cut into 4 µm sections. Sections were stained with TRITC-conjugated goat anti-mouse IgG and FITC-conjugated donkey anti-mouse IgM (both 1:100 diluted, Jackson ImmunoResearch Laboratories) for 30 min at 37 °C. Immunofluorescence staining intensity of glomeruli was scored on a scale of 0–3 under inverted fluorescence microscope (Olympus).
Flow cytometry
The following anti-mouse Abs were used to characterize spleen cell subsets: Th1/Th2 (FITC-anti-CD4, PE-anti-IL4, APC-anti-IFN-γ); Treg (FITC-anti-CD4, PE-anti-Foxp3, APC-anti-CD25); Th17 (FITC-anti-CD4, APC-anti-IL17A); Tfh (FITC-anti-CD4, PE-anti-PD-1, APC-anti-CXCR5); plasma cells (FITC-anti-B220, PE-anti-CD138). For intracellular cytokine staining, cells were stimulated with 50 ng/mL phorbol 12-myristate 13-acetate (PMA) plus 1 µg/mL ionomycin and 5 µg/mL brefeldin A at 37 °C for 4 hours. All antibodies were purchased from eBioscience. Data were acquired by flow cytometer (Calibur, BD Biosciences, CA, USA) and analyzed with FlowJo software (Tree Star, USA).
Measurement of serum cytokines
Measurement of IL-6, IL-10, IL-17, MCP-1 in serum was carried out by using Milliplex MAP technology. Immunology multiplex assay kit were obtained from MERCK, and the procedure was performed following the manufacturer’s instruction. Results were acquired by the MILLIPLEX Analyst.V5.1 software and the standard curves were plotted through a five-parameter logistic curve setting.
Statistical analysis
All data are expressed as the mean ± SEM. One-way ANOVA, Kruskal-Wallis and Mann-Whitney U test were used to assess significance between group comparisons using SPSS 22.0 software. Correlations were evaluated with a two-tailed Pearson correlation test by GraphPad Prism 5 software. P values less than 0.05 were considered statistically significant.
Results
Characteristics of DPSCs and PDLSCs
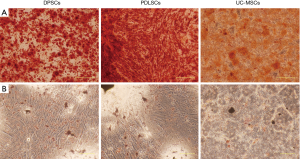
DPSCs, PDLSCs and UC-MSCs showed similar expression profile of cell surface markers (Figure 1). All the three types of MSCs were strongly positive for CD44, CD90 (stromal cells surface markers), CD73 and CD105 (cell surface markers associated with stromal cells and endothelial cells), but negative for CD34 (hematopoietic cell marker), CD14 (cell surface marker for monocytes and macrophages), CD45 (leukocyte marker), CD79 (B cells marker), HLA-DR (antigen presenting cells marker). CD106 (VCAM1) is a cytokine-inducible cell surface protein capable of mediating adhesion. Previous studies showed that CD106 was critical for MSC-mediated immunosuppression (15,16). We found that DPSCs showed higher expression of CD106 than UC-MSCs and PDLSCs. Under appropriate conditions both DPSCs and PDLSCs can differentiate into adipocytes and osteocytes (Figure S1).

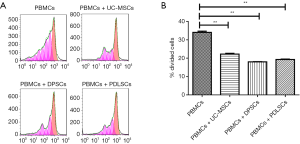
Inhibitory effects of dental MSCs on the PBMC proliferation
To examine whether MSCs from dental tissue exhibit any difference in inhibiting the proliferation of PBMCs, we compared their capability of suppressing PBMC proliferation. As shown in Figure 2, UC-MSCs, PDLSCs and DPSCs showed comparable capacity of suppressing PBMC proliferation.
MSCs transplantation alleviated SLE-like symptoms in B6/lpr mice
Our previous study showed that infusion of BM-MSCs or UC-MSCs could effectively alleviate disorders in SLE patients and mice (12,17-20). Here we employed B6/lpr mice to evaluate the therapeutic effects of dental tissue-derived MSCs in SLE (Figure 3A). There was no significant difference in the changes of body weight and spleen index (spleen weight/body weight) among groups (Figure 3B,C). But the lymph node (LN) indexes (LN weight/body weight) of mice treated with UC-MSCs and PDLSCs dramatically decreased (Figure 3D).

Next, we sought to determine whether treatment with MSCs could reduce the renal injury in B6/lpr mice, 24-h urine was collected every 3 weeks after treatment and urine albumin excretion was determined by urine protein test kit (Nanjing Jiancheng Bioengineering Institute). We found that mice transplanted with DPSCs or PDLSCs had less proteinuria at both 6 and 9 weeks than PBS (Figure 3E).
It is known that autoantibodies play a crucial role in pathogenesis of SLE. Our previous study showed that anti-double strand DNA (anti-dsDNA) antibody and anti-nuclear antibody (ANA) were remarkable increased in peripheral blood of C3H/lpr and MRL/lpr mice (17,18). As seen in UC-MSCs transplantation, PDLSCs and DPSCs transplantation also resulted in a significant reduction in serum anti-dsDNA antibody, while there was no significant difference of serum levels of ANA compared to PBS group (Figure 3F,G).
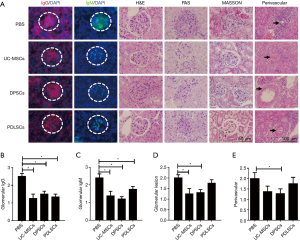
Dental MSCs improved kidney pathology of lupus mice
We then assessed the histopathological changes in kidneys of B6/lpr mice received PDLSCs, PDLSCs or UC-MSCs. IgG and IgM deposition in the glomeruli decreased in PDLSCs and DPSCs treated mice compared with PBS group (Figure 4A,B,C). However, glomerular pathology was not completely improved by MSCs treatment. There was a reduction in glomerular hypercellularity in DPSCs group, but not in PDLSCs group (Figure 4D). The extent of perivascular pathology, the number of cell layers surrounding the vessel walls, only diminished in the DPSCs treated mice compared with the control group (Figure 4E). The histological analysis with PAS and MASSON staining did not show any obvious abnormality in all groups (Figure 4A), which was possible attributed to slight glomerulonephritis of B6/lpr (21).
MSCs transplantation inhibited Th1 and plasma cells
Accumulating data showed that Th1 cytokines, especially interferon-γ (IFNγ), were responsible for pathogenesis of lupus (22). Treg and Th17 cells were also reported to play roles in disease process of MRL/lpr mice (17). Additionally, an increase of Tfh cells, which help B cell to activate and produce autoantibody, has been noticed in peripheral blood from SLE patients (23). In our experiment, we just found the ratios of Th1, Tfh and plasma cells were higher in the spleens of B6/lpr than B6 mice, while there is no difference of Th2, Treg and Th17 in the spleens between the two groups (Figure S2). Despite these, we next examined the changes of Th1, Th2, Th17, Tfh, Treg and plasma cells in the spleen of B6/lpr mice 10 weeks after MSCs transplantation. As shown in Figure 5A,B,C, compared with PBS group, CD4+ T cells producing IFN-γ, but not IL4, significantly decreased in B6/lpr mice treated with PDLSCs and DPSCs. No difference was observed in the percentages of Treg and Th17 cells between the two dental tissue-derived MSCs groups (Figure 5D,E,F,G). No changes of Tfh cells after MSCs transplantation have been observed (Figure 5H,I). But for plasma cells, they were significantly reduced by PDLSCs and DPSCs (Figure 5J,K).


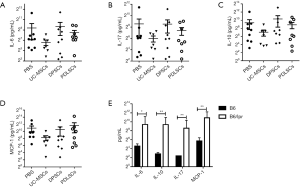
Influences of MSCs on serum cytokines of B6/lpr mice
Serum IL-6, IL-10, IL-17 and MCP-1 levels were dramatically elevated in active SLE patients and positively correlated with the disease activity index (24-26). We next examined whether MSCs could downregulate these inflammatory cytokines. As expected, all of the cytokines mentioned above were dramatically increased in B6.lpr mice. Unfortunately, we didn’t find any statistical differences of IL-6, IL-10, IL-17 and MCP-1 among PDLSCs, DPSCs and PBS treated mice (Figure 6). To evaluate which cytokine was associated with nephritis, we analyzed the correlation of cytokines with proteinuria and anti-dsDNA and no statistically significant association was found (P>0.05; data not shown).
Discussion
MSCs has been proposed for refractory SLE treatment with little adverse reactions. MSCs can be isolated from various tissues, including BM, UC, AD. Recently, DPSCs and PDLSCs derived from a very accessible tissue resource have been reported as substitute for treating various disease and repairing tissue (10,11).
In present study, DPSCs and PDLSCs were positive for mesenchymal stem cell markers, including CD44, CD73, CD90, CD105, and expression pattern was similar to previous reports (27). While they were negative for CD45 and CD34 which were hematopoietic stem cell makers. These results suggest that the DPSCs and PDLSCs were mesenchymal stem cells and not hematopoietic stem cell lineage. Interestingly, we found DPSCs express higher level of CD106 than UC-MSCs and PDLSCs. It has been reported that CD106 positive MSCs with powerful immunosuppressive activity (16). Therefore, we speculate that DPSCs may obtain better immunosuppressive functions than PDLSCs.
The results of the present study showed that transplantation of DPSCs and PDLSCs alleviated the SLE symptoms of B6.lpr mice. Both of the two cells were able to reduce the proteinuria, serum anti-dsDNA antibodies, glomerular IgG and IgM; however, it was the DPSCs not PDLSCs that relieved glomerular lesion and reduced perivascular cell accumulation.
It has been well-accepted that T-cell abnormality plays an essential role in the development of SLE, but which type of the Th cells plays a dominant role is unclear (28-30). During the past several decades, IFN-γ was recognized as critical pathogenic factor in human and murine lupus (29,31,32). Recently, IL-17 was also reported to play an important role in pathogenesis of SLE. In our study, we carefully examined the Th1, Th2, Th17 cells, Tfh and Treg cells of spleen from B6/lpr mice received UC-MSCs, DPSCs and PDLSCs therapy. We found that DPSCs and PDLSCs transplantation had no effect on Treg, Th17 and Tfh in B6/lpr mice. But infusion of DPSCs and PDLSCs significantly down-regulated Th1 cells as well as UC-MSCs. Yuan et.al reported that human MSCs could inhibit the differentiation of Th17 cells in MRL/lpr mice (33), however, in our study we did not find any change of the proportion of Th17 cells in the spleen of B6/lpr mice after treated with human MSCs. This might due to the difference in the time to give MSCs.
IL-6, which promotes the development of Th17 cells, is higher in SLE patients than health subjects. Several studies reported that IL-6 played an important role in the B-cell hyperactivity, auto-antibodies production and immunopathology of SLE (34). IL-10 was also found significantly higher in plasma of SLE patients (25). It was over-produced by B cells and monocytes and played a central role in the pathogenesis of SLE including regulation of growth and differentiation of B cells and auto-antibody production. Thus, IL-10 has recently been suggested as a biomarker of SLE (24). Monocyte chemoattractant protein-1 (MCP-1/CCL2) is one of the key chemokines that regulate migration and infiltration of monocytes/macrophages. In SLE patients without renal disease MCP-1 correlated positively with SLEDAI score, but negatively in those with renal derangement (35). In the present study, we did not find significant reduction of IL-6, IL-17, IL-10 and MCP-1 in serum of MSCs treated B6/lpr mice compared with those in controls. So, in our experiment, MSCs ameliorate the SLE symptom of B6/lpr may not through regulating the cytokines.
Conclusions
In conclusion, our study demonstrated that, like UC-MSCs, PDLSCs and DPSCs also have effects in ameliorating disease symptoms in lupus-prone B6/lpr mice. Moreover, DPSCs show better effect on reducing kidney glomerular lesion and perivascular inflammation infiltration than PDLSCs.
Acknowledgements
Funding: This work was supported by grant from National Natural Science Foundation (81501395), Jiangsu Province Natural Science Foundation (BK20160115), the Major International (Regional) Joint Research Project (81720108020), Jiangsu Province Major Research and Development Program (BE2015602), Jiangsu Province 333 Talent Grant (BRA2016001), China.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethics Committee for Animal Research in the Affiliated Drum Tower Hospital of Nanjing University Medical School (IRB number: 20150501).
References
- Flores-Mendoza G, Sanson SP, Rodriguez-Castro S, et al. Mechanisms of Tissue Injury in Lupus Nephritis. Trends Mol Med 2018;24:364-78. [Crossref] [PubMed]
- Tsokos GC. Systemic lupus erythematosus. N Engl J Med 2011;365:2110-21. [Crossref] [PubMed]
- Felten R, Dervovic E, Chasset F, et al. The 2018 pipeline of targeted therapies under clinical development for Systemic Lupus Erythematosus: a systematic review of trials. Autoimmun Rev 2018;17:781-90. [Crossref] [PubMed]
- Ma H, Liu C, Shi B, et al. Mesenchymal Stem Cells Control Complement C5 Activation by Factor H in Lupus Nephritis. EBioMedicine 2018;32:21-30. [Crossref] [PubMed]
- Wang D, Niu L, Feng X, et al. Long-term safety of umbilical cord mesenchymal stem cells transplantation for systemic lupus erythematosus: a 6-year follow-up study. Clin Exp Med 2017;17:333-40. [Crossref] [PubMed]
- Jang E, Jeong M, Kim S, et al. Infusion of Human Bone Marrow-Derived Mesenchymal Stem Cells Alleviates Autoimmune Nephritis in a Lupus Model by Suppressing Follicular Helper T-Cell Development. Cell Transplant 2016;25:1-15. [Crossref] [PubMed]
- Thiel A, Yavanian G, Nastke MD, et al. Human embryonic stem cell-derived mesenchymal cells preserve kidney function and extend lifespan in NZB/W F1 mouse model of lupus nephritis. Sci Rep 2015;5:17685. [Crossref] [PubMed]
- Yamaza T, Kentaro A, Chen C, et al. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther 2010;1:5. [Crossref] [PubMed]
- Choi EW, Shin IS, Park SY, et al. Reversal of serologic, immunologic, and histologic dysfunction in mice with systemic lupus erythematosus by long-term serial adipose tissue-derived mesenchymal stem cell transplantation. Arthritis Rheum 2012;64:243-53. [Crossref] [PubMed]
- Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 2000;97:13625-30. [Crossref] [PubMed]
- Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004;364:149-55. [Crossref] [PubMed]
- Gu Z, Akiyama K, Ma X, et al. Transplantation of umbilical cord mesenchymal stem cells alleviates lupus nephritis in MRL/lpr mice. Lupus 2010;19:1502-14. [Crossref] [PubMed]
- Chan OT, Hannum LG, Haberman AM, et al. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med 1999;189:1639-48. [Crossref] [PubMed]
- Kikawada E, Lenda DM, Kelley VR. IL-12 Deficiency in MRL-Faslpr Mice Delays Nephritis and Intrarenal IFN- Expression, and Diminishes Systemic Pathology. J Immunol 2003;170:3915-25. [Crossref] [PubMed]
- Ren G, Zhao X, Zhang L, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol 2010;184:2321-8. [Crossref] [PubMed]
- Yang ZX, Han ZB, Ji YR, et al. CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties. PLoS One 2013;8:e59354. [Crossref] [PubMed]
- Sun L, Akiyama K, Zhang H, et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells 2009;27:1421-32. [Crossref] [PubMed]
- Ma X, Che N, Gu Z, et al. Allogenic mesenchymal stem cell transplantation ameliorates nephritis in lupus mice via inhibition of B-cell activation. Cell Transplant 2013;22:2279-90. [Crossref] [PubMed]
- Wang D, Li J, Zhang Y, et al. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study. Arthritis Res Ther 2014;16:R79. [Crossref] [PubMed]
- Liang J, Sun L. Mesenchymal stem cells transplantation for systemic lupus erythematosus. Int J Rheum Dis 2015;18:164-71. [Crossref] [PubMed]
- Izui S, Kelley VE, Masuda K, et al. Induction of various autoantibodies by mutant gene lpr in several strains of mice. J Immunol 1984;133:227-33. [PubMed]
- Balomenos D, Rumold R, Theofilopoulos AN. Interferon-gamma is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. J Clin Invest 1998;101:364-71. [Crossref] [PubMed]
- Xu H, Liu J, Cui X, et al. Increased frequency of circulating follicular helper T cells in lupus patients is associated with autoantibody production in a CD40L-dependent manner. Cell Immunol 2015;295:46-51. [Crossref] [PubMed]
- Chun HY, Chung JW, Kim HA, et al. Cytokine IL-6 and IL-10 as biomarkers in systemic lupus erythematosus. J Clin Immunol 2007;27:461-6. [Crossref] [PubMed]
- Mok MY, Wu HJ, Lo Y, et al. The relation of interleukin 17 (IL-17) and IL-23 to Th1/Th2 cytokines and disease activity in systemic lupus erythematosus. J Rheumatol 2010;37:2046-52. [Crossref] [PubMed]
- Deshmane SL, Kremlev S, Amini S, et al. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 2009;29:313-26. [Crossref] [PubMed]
- Wada N, Menicanin D, Shi S, et al. Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol 2009;219:667-76. [Crossref] [PubMed]
- Wong CK, Ho CY, Li EK, et al. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus 2000;9:589-93. [Crossref] [PubMed]
- Takahashi S, Fossati L, Iwamoto M, et al. Imbalance towards Th1 predominance is associated with acceleration of lupus-like autoimmune syndrome in MRL mice. J Clin Invest 1996;97:1597-604. [Crossref] [PubMed]
- Piantoni S, Andreoli L, Scarsi M, et al. Phenotype modifications of T-cells and their shift toward a Th2 response in patients with systemic lupus erythematosus supplemented with different monthly regimens of vitamin D. Lupus 2015;24:490-8. [Crossref] [PubMed]
- Masutani K, Akahoshi M, Tsuruya K, et al. Predominance of Th1 immune response in diffuse proliferative lupus nephritis. Arthritis Rheum 2001;44:2097-106. [Crossref] [PubMed]
- Akahoshi M, Nakashima H, Tanaka Y, et al. Th1/Th2 balance of peripheral T helper cells in systemic lupus erythematosus. Arthritis Rheum 1999;42:1644-8. [Crossref] [PubMed]
- Yuan L, Xiao ZT, Huang XZ, et al. Human embryonic mesenchymal stem cells alleviate pathologic changes of MRL/Lpr mice by regulating Th7 cell differentiation. Ren Fail 2016;38:1432-40. [Crossref] [PubMed]
- Talaat RM, Mohamed SF, Bassyouni IH, et al. Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: Correlation with disease activity. Cytokine 2015;72:146-53. [Crossref] [PubMed]
- Lit LC, Wong CK, Tam LS, et al. Raised plasma concentration and ex vivo production of inflammatory chemokines in patients with systemic lupus erythematosus. Ann Rheum Dis 2006;65:209-15. [Crossref] [PubMed]


