
New tools for Wilson’s disease diagnosis: exchangeable copper fraction
In Wilson’s disease (WD), irreversible and serious clinical injuries and even death could be consequences of misdiagnosis or treatment delay (1). The diagnosis is evoked on clinical symptoms and several biological tests: ceruloplasmin (Cp), serum and urinary copper concentrations, and sometimes calculated estimation of copper that is not bound to Cp. These determinations are often insufficient to diagnose or to exclude WD. Although hepatic copper evaluation is rather discriminatory and helps for diagnosis, invasive sampling, false negative results and inhomogeneous liver copper distribution moderate its usefulness (2). Molecular genetic diagnosis confirms the diagnosis in 98% of cases; the result is that 2% of patients with proven WD have a single heterozygous mutation or even no mutation in the ATP7B gene (3); furthermore, this diagnosis can be time-consuming because of more than 600 mutations have been described. However, early diagnosis and treatment are the keystones of the successful management of patients with WD. But thinking of a WD diagnosis is challenging as many patients have non-specific symptoms that precede those leading to the diagnosis. So, it is important to have a rapid, reliable, non-invasive and discriminative tool for the diagnosis of WD. It is why we developed the exchangeable copper (CuEXC) assay that leads to the calculation of the relative exchangeable copper (REC).
The limits of the traditional and cupric parameters
The traditional and non-invasive cupric parameters used for WD diagnosis are serum Cp and copper concentrations, calculated non-Cp-bound copper and 24 h-urinary copper excretion.
Serum Cp determination
Serum copper is mostly carried by Cp. This protein is synthesized in hepatic microsomes as an inactive, unstable non-copper-bound apoprotein called apo-Cp. Charged with six copper atoms, it is excreted in the circulation as a holoprotein with acts as a ferro-oxidase (4). In normal subjects, 90% of plasma Cp circulates as holo-Cp and therefore contains copper.
The determination of serum Cp is essentially performed by immunological method (as radioimmunoassay, radial immunodiffusion or nephelometry) which simultaneously measures inactive apo-Cp and active holo-Cp. This method currently used by automated clinical laboratories is rapid but tends to overestimate active Cp. The only method available to determine the copper-dependent oxidative activity of Cp (active holo-Cp) is an enzymatic determination that is not performed routinely.
Due to ATP7B defect in WD, apo-Cp copper incorporation is stucked, and holo-Cp serum concentration is decreased while apo-Cp and free copper are released from liver. Serum Cp is typically decreased in WD, lower than 0.1 g/L. But diagnosis couldn’t be excluded in front of normal Cp observation, more than half of patients presenting severe liver disease (5), 25–36% of children with WD (6) and few patients with neurologic presentation of WD (7) have normal serum Cp measured by immunologic assays. Patients with sub-normal levels of Cp during the acute phase are likely to synthesize apo-Cp essentially. Cp enzymatic activity determination has then been proposed (8,9). These enzymatic determinations detect only active holo-Cp and are the reflect of what ATP7B does in hepatocytes. Methods for Cp enzymatic activity determination, instead of immunological antigenic properties, rely on functionally active Cp catalytic oxidation, towards different substrates. ρ-phenylenediamine (PPD) was the first described (10). Ortho-dianisidine dihydrochloride (OD) was also used in numerous methods (11,12). The use of ferric iron, considered as the only biological substrate, allows to determine Cp ferroxidase activity (13,14). These assays, even applied to automated analyzers, are not yet available in routine, but will certainly be more informative for WD diagnosis than immunological assay.
In addition to these methodological problems, there are physiological variations in Cp levels that can mislead to diagnosis. Inflammation states, pregnancy or estrogen supplementation can lead to serum Cp elevation (15). On the other hand, low serum Cp levels are observed in newborns or during Menkes disease, aceruloplasminemia, nephritic syndrome, copper deficiencies, severe chronic liver disease or malabsorption syndromes (16) and, about 20% of heterozygous subjects for the WD gene have reduced levels (17). Therefore, serum Cp interpretation is not easy and insufficient for WD diagnosis.
Serum copper
Circulating copper is partly inextricably bound within metalloprotein (Cp essentially) or loosely bound to proteins such as albumin, amino acids or peptides. Physiologically, nearly 70% of copper is bound to Cp and less than 20% is bound to albumin. Transcuprein (αmacroglobulin) and amino acids binds 7–15% and 2–5% of serum copper, respectively (18). Total serum copper determination measures copper incorporated in Cp and non-Cp bound copper. It is usually made by either atomic absorption or emission spectrometry or by inductively coupled plasma mass spectrometry (ICP-MS) methods (19). Normal range of total serum copper is estimated around 12.7–22. 2 µmol/L.
Although considered as a copper overload, total serum copper and Cp are usually decreased in WD. Indeed, the absence of holo-Cp that carries copper atoms, leads to dramatical reduction of total serum copper. However, normal serum copper concentration can be observed in WD. Acute hemolysis or hepatitis can lead to important release of copper from liver tissue stores. Dissociation between normal to increased total copper and decreased Cp levels could indicate an increase in the non-Cp-bound-copper. So, it’s important to differentiate copper highly bound to Cp that is not bioavailable for tissues and organs from labile or free copper pool which, in case of WD, is thought to be responsible of organ damage.
Calculated non-Cp-bound copper concentration (NCC)
By estimating the toxic unbound (or “free”) copper, NCC was proposed as a diagnostic test for WD (20,21). NCC is calculated from Cp and total serum copper concentrations and the adequacy of both measurements influences results of the formula used for calculation (22). In fact, this determination’s limitations in WD context are due to very low values of total copper and Cp which sometimes do not reach analytical method detection limits. Furthermore, this calculation is not valid when immunological assay is used for serum Cp determination, because of simultaneous detection of inactive apo-Cp and active holo-Cp. Oxidase activity measurement of Cp could resolve these difficulties but this enzymatic determination is not performed routinely.
Several authors showed that NCC is not a good test in WD. Twomey et al. showed large overlapping of this parameter between non WD subjects and WD patients. Moreover, due to Cp overestimation, nearly 20% of normal subjects present negative values (23). Our team showed also that 10.4% of WD patients have negative values of NCC at diagnosis (24). European Association for the Study of the Liver (EASL) guidelines did not recommend NCC for diagnosis of WD (25).
24 h-urinary copper excretion
Basal 24-h urinary copper excretion reflects, in non-treated patients, the amount of non-bound circulating copper. In symptomatic, non-treated patients, a threshold of 100 µg (1.6 µmol)/24 hours urinary copper excretion, has been retained for WD diagnosis (26). In practice, knowing the exact 24 hours urine volume can be difficult in young children and in patients with neurological symptoms. In addition, care should be taken to avoid external contaminations, which are very common for urine collections (27). At last, this determination is not applicable in case of renal impairment (28). Interpreting 24-h urinary copper excretion may be difficult. At the time of diagnosis, especially in children and asymptomatic siblings, more than a quarter of WD patients have 24-h urinary copper levels below the threshold described (29). So, the lower cut-off of 0.6 µmol/24 hours is considered to be more sensitive (30,31). Furthermore, high urinary copper excretion may be difficult to interpret, particularly because of the increasing observed in different liver diseases (32). Heterozygotes subjects on ATP7B gene may also present increased urinary copper level (5). Urinary copper excretion measurement under D-penicillamine treatment as a diagnosis test has been proposed but reference values have only been validated in children with liver symptoms (29).
In summary
Each isolated traditional biochemical marker is insufficient to set WD diagnosis. The association of the three biochemical markers (low serum Cp concentration, low serum copper concentration and high urinary copper excretion) is highly predictible of WD diagnosis. However, this classical triad is present in 15% of heterozygote carrier and 3% of WD patients with confirmed mutations have normal copper balance (personal data, Lariboisiere registry). Calculation of NCC is flawed. So, we focused our research on a direct determination of the labile fraction of serum copper.
Direct plasma unbound copper determination
We distinguish ultrafiltrable copper (CuUF) and CuEXC. The results of recent studies with quantification of CuUF and CuEXC in WD animal models and human populations are summarized in Table 1.
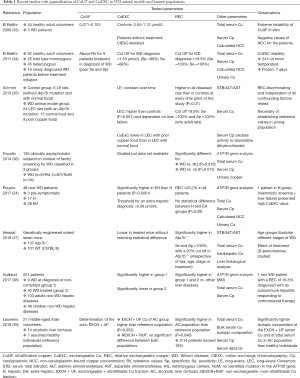
Full table
Plasma CuUF
This fraction of copper which is bound to low molar mass molecules is obtained by ultrafiltration of plasma through a membrane with a cut off retaining albumin (67 kDa), Cp (132 kDa), and transcuprein (270 kDa). CuUF represents less than 1% of total copper in healthy subjects and is supposed to be constituted by copper released from liver that is bound to proteins such as albumin. CuUF is an unstable fraction that changes with copper movements between free form and plasma proteins binding (33). Moreover, CuUF have not demonstrated a great value in the diagnosis of WD patients (34).
CuEXC, a marker of the dissemination and severity of WD
This other fraction is obtained after albumin and amino acids copper complexation. Heavy extraction procedures have sometimes been used (40-42). However, easier complexation procedures using chelators such as ethylenediaminetetraacetic acid (EDTA) are able to mobilize this exchangeable fraction. Retained procedure involves serum ultrafiltration after 1-hour EDTA incubation. CuEXC includes both CuUF and copper loosely bound to albumin and other amino acids and then constitutes non-Cp-bound copper.
This determination has shown a good analytical reliability and a 24 hours or 7 days stability at room temperature or in frozen serum respectively (34). Thus, CuEXC determination requires an immediate serum freezing after sampling centrifugation if conservation exceed 24 h. Reference values for CuEXC are between 0.62 and 1.15 µmol/L (18).
Main advantage of this assay is that it doesn’t depend on the dosage of Cp and it represents an exact estimation of copper overload. If CuEXC is over reference range then a blood and tissue copper overload are suspected as consequence of hepatocytes saturation and leakage of toxic free copper into the blood.
Studies in WD animal models showed that CuEXC increases with liver disease evolution. Long Evans Cinnamon (LEC) rats present, as a consequence of a loss of function mutation in the ATP7B gene a copper metabolism disorder. Schmitt et al. demonstrated that, in these rats, CuEXC levels were correlated with acute liver disease (35). In another WD animal model (mice from a genetically engineered rodent strain), Atp7b−/− mice have several features of WD. In these animals, hepatic lesions and CuEXC increase are observed, even if no acute liver failure was observed in this study (37).
We demonstrated that in case of WD affecting only the liver at diagnosis, CuEXC is normal or moderately increased except in case of acute liver failure associated with Coombs negative haemolytic failure. In these cases, the increase of CuEXC is owing to hepatic necrosis (24). Moreover, the level of CuEXC is shown to be statistically higher in WD patients with extrahepatic involvement (Kayser-Fleischer ring, neurological symptoms, pathological brain MRI) than in hepatic WD. A threshold of 2.08 µmol/L permitted to predict the presence of these extrahepatic lesions. Furthermore, CuEXC level at diagnosis time is predictive of extrahepatic involvement severity: a positive correlation is observed between CuEXC and the Unified Wilson Disease Rating Scale (UWDRS) (24). UWDRS neurological score, specific for WD has been developed for clinical studies and is widely used by WD expert teams. It evaluates consciousness (part 1), functional scale based on Barthel scale and activities of daily living (part 2) and neurological examination: speech, gait, and dystonic, ataxic, tremor and parkinsonian syndromes (part 3) (43). Interestingly, sensitivity to dietary copper has been demonstrated in LEC rats WD animal models (44) with lower CuEXC in low-copper diet rats (35). CuEXC is also interesting for WD treatment observance evaluation, with higher levels in non-compliant patients (38). In a population study of 100 WD patients, CuEXC abnormal increase was observed in 25 patients reflecting compliance/observance problems. Half of them (12/25) showed also liver enzymes increase. These observations highlighted the ability of CuEXC to reflect copper overload.
CuEXC is not yet referenced in French bio-clinical analysis nomenclature. The analysis cost depends on the analytical methodology used for copper determination in the ultra-filtrate: Atomic Absorption Spectroscopy (AAS) or ICP-MS. It may, then, be slightly variable from a laboratory to another. In addition, the Ultra centrifugal filter (Amicon®) unit’s cost has also to be included, and as REC determination also involves total serum copper determination, this parameter has to be taken in account for analysis billing.
REC: a specific and sensitive non-invasive tool for WD diagnosis
The percentage of exchangeable to total serum copper (e.g., copper incorporated in Cp and non-Cp-bound copper) called the REC appreciates toxic blood copper fraction. It is the most informative non-invasive WD diagnosis test. In an original work, El Balkhi et al. compared the REC to classical WD diagnosis tests (total serum copper, urinary copper excretion, Cp and NCC) in three populations (WD patients, heterozygous individuals and normal subjects). REC evaluation with an 18.5% cut off was shown to be more sensitive and more specific than other usual tests. REC test is then an excellent biomarker for the diagnosis of WD with 100% sensitivity and 100% specificity (34). These results were also confirmed in LEC rats; Schmitt et al. tested the validity of REC, in comparison with Cp oxidase activity and total serum copper, in different stages of liver disease. Using a cut off slightly higher than human threshold (19%), REC was the only parameter that permitted to distinguish between controls (Long-Evans rats) and WD models (LEC rats) with a 100% sensitivity and specificity. In addition, the authors demonstrated that liver failure stage didn’t influence REC level (35). In another animal model, Atp7b−/− mice, discriminative power of REC was also demonstrated for WD diagnosis: diseased group mice presented an average REC statistically higher than wild type group. Here again, sensitivity and specificity of REC was fined to 100% using a cut off fixed at 20% (37).
We have also shown that REC is particularly efficient to distinguish individuals carrying one abnormal ATP7B allele from WD patients. This study was conducted in 127 asymptomatic siblings (up to the second-degree relatives) of index case WD patients presenting a genetically confirmed diagnosis. With a cut-off of 15%, REC determination significantly discriminated WD patients from individuals carrying one abnormal ATP7B allele and normal subjects (36). REC is then an important parameter for WD family screening, that can also discriminate carriers (presenting heterozygous mutation in ATP7B gene) even in presence of copper biological abnormalities.
Moreover, REC performance in Wilson’s disease diagnosis has been studied in a study group counting 103 adults and 49 children with various kinds of chronic liver disease (excluding WD) (38). The discriminative performance of REC, for WD diagnosis, has also been demonstrated among non-Wilsonian liver diseases patients (REC >18.5%, sensitivity and specificity of 100%) in addition to previously published discrimination among controls, heterozygous carriers, and family relatives (34,36), else more the authors noticed that in the other chronic liver diseases tested while liver function tests were abnormal REC remained normal and did not increase. In cirrhosis, because of frequent low serum Cp, REC is particularly interesting (45) and in icteric cholestasis because of basal 24-hour urine copper excretion frequently increased in this disorder (32). Nevertheless, in a recent study, 14 patients with alcoholic cirrhosis subjects have REC value ≤19% except two patients with REC at 21 and 25%. There is no information concerning ATP7B genetic testing for these patients (39).
Conclusions
CuEXC determination allows a direct an accurate measurement of copper overload. It provides, at diagnosis, information on the spread and severity of the disease. REC calculation (percentage of exchangeable to total serum copper) is a very valuable discriminative tool for WD diagnosis presenting excellent sensitivity and specificity. These two tests are rapid, reliable and non-invasive. Their routine uses, by different teams, in large cohorts of patients have been very successful and validated their important place as standard of care for WD patients. They give fast answers to an appropriate diagnosis and allow beginning quickly the treatment without waiting for genetic testing results. Furthermore, they proved a helpful contribution in initial treatment choice and dose progression rate determination.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Walshe JM. Cause of death in Wilson disease. Mov Disord 2007;22:2216-20. [Crossref] [PubMed]
- Ferenci P, Steindl-Munda P, Vogel W, et al. Diagnostic value of quantitative hepatic copper determination in patients with Wilson’s Disease. Clin Gastroenterol Hepatol 2005;3:811-8. [Crossref] [PubMed]
- Collet C, Woimant F, Laplanche JL, et al. ADN: études génétiques en vue du diagnostic de maladie de Wilson. EMC - Biologie médicale (Paris) 2019;14:1-6.
- Bielli P, Calabrese L. Structure to function relationships in ceruloplasmin: a 'moonlighting' protein. Cell Mol Life Sci 2002;59:1413-27. [Crossref] [PubMed]
- Steindl P, Ferenci P, Dienes HP, et al. Wilson's disease in patients presenting with liver disease: a diagnostic challenge. Gastroenterology 1997;113:212-8. [Crossref] [PubMed]
- Sánchez-Albisua I, Garde T, Hierro L, et al. A high index of suspicion: the key to an early diagnosis of Wilson's disease in childhood. J Pediatr Gastroenterol Nutr 1999;28:186-90. [Crossref] [PubMed]
- Woimant F, Trocello JM. Disorders of heavy metals. Handb Clin Neurol 2014;120:851-64. [Crossref] [PubMed]
- Macintyre G, Gutfreund KS, Martin WRW, et al. Value of an enzymatic assay for the determination of serum ceruloplasmin. J Lab Clin Med 2004;144:294-301. [Crossref] [PubMed]
- Merle U, Eisenbach C, Weiss KH, et al. Serum ceruloplasmin oxidase activity is a sensitive and highly specific diagnostic marker for Wilson's disease. J Hepatol 2009;51:925-30. [Crossref] [PubMed]
- Sunderman FW, Nomoto S. Measurement of human serum Ceruloplasmin by its p-phenylenediamine oxidase activity. Clin Chem 1970;16:903-10. [PubMed]
- Schosinsky KH, Lehmann HP, Beeler MF. Measurement of ceruloplasmin from its oxidase activity in serum by use of o-dianisidine dihydrochloride. Clin Chem 1974;20:1556-63. [PubMed]
- Siotto M, Pasqualetti P, Marano M, et al. Automation of o-dianisidine assay for ceruloplasmin activity analyses: usefulness of investigation in Wilson’s disease and in hepatic encephalopathy. J Neural Transm 2014;121:1281-6. [Crossref] [PubMed]
- Erel O. Automated measurement of serum ferroxidase activity. Clin Chem 1998;44:2313-9. [PubMed]
- Neselioglu S, Ergin M, Erel O. A New Kinetic, automated assay to determine the ferroxidase activity of ceruloplasmin. Anal Sci 2017;33:1339-44. [Crossref] [PubMed]
- Danzeisen R, Araya M, Harrison B, et al. How reliable and robust are current biomarkers for copper status? Br J Nutr 2007;98:676-83. [Crossref] [PubMed]
- Poujois A, Djebrani-Oussedik N, Ory-Magne F, et al. Neurological presentations revealing acquired copper deficiency: diagnosis features, aetiologies and evolution in seven patients. Intern Med J 2018;48:535-40. [Crossref] [PubMed]
- Gromadzka G, Chabik G, Mendel T, et al. Middle-aged heterozygous carriers of Wilson's disease do not present with significant phenotypic deviations related to copper metabolism. J Genet 2010;89:463-7. [Crossref] [PubMed]
- Poujois A, Poupon J, Woimant F. Direct determination of non-ceruloplasmin-bound copper in plasma. In: Kerkar N, Eve A. editors. Clinical and translational perspectives on Wilson disease. Elsevier, 2019:249-55.
- McMillin GA, Travis JJ, Hunt JW. Direct measurement of free copper in serum or plasma ultrafiltrate. Am J Clin Pathol 2009;131:160-5. [Crossref] [PubMed]
- Twomey PJ, Viljoen A, Reynolds TM, et al. Non-ceruloplasmin-bound copper in routine clinical practice in different laboratories. J Trace Elem Med Biol 2008;22:50-3. [Crossref] [PubMed]
- Walshe JM. Monitoring copper in Wilson's disease. Adv Clin Chem 2010;50:151-63. [Crossref] [PubMed]
- Ferenci P. Diagnosis of Wilson disease. Handb Clin Neurol 2017;142:171-80. [Crossref] [PubMed]
- Twomey PJ, Viljoen A, House IM, et al. Limitations of non-ceruloplasmin-bound copper in routine clinical practice. Gut 2007;56:154. [PubMed]
- Poujois A, Trocello JM, Djebrani-Oussedik N, et al. Exchangeable copper: a reflection of the neurological severity in Wilson's disease. Eur J Neurol 2017;24:154-60. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Wilson’s disease. J Hepatol 2012;56:671-85. [Crossref] [PubMed]
- Tu JB, Blackwell RQ. Studies on levels of penicillamine-induced cupriuresis in heterozygotes of Wilson's disease. Metabolism 1967;16:507-13. [Crossref] [PubMed]
- Pineau A, Guillard O, Chappuis P, et al. Sampling conditions of biological fluids for monitoring trace elements at hospital: a practical review. Crit Rev Clin Lab Sci 1993;30:203-22. [Crossref] [PubMed]
- Zhang T, Chang X, Liu W, et al. Comparison of sodium, potassium, calcium, magnesium, zinc, copper and iron concentrations of elements in 24-h urine and spot urine in hypertensive patients with healthy renal function. J Trace Elem Med Biol 2017;44:104-8. [Crossref] [PubMed]
- Müller T, Koppikar S, Taylor RM, et al. Re-evaluation of the penicillamine challenge test in the diagnosis of Wilson's disease in children. J Hepatol 2007;47:270-6. [Crossref] [PubMed]
- Gow PJ, Smallwood RA, Angus PW, et al. Diagnosis of Wilson’s disease: an experience over three decades. Gut 2000;46:415-9. [Crossref] [PubMed]
- García-Villarreal L, Daniels S, Shaw SH, et al. High prevalence of the very rare Wilson disease gene mutation Leu708Pro in the Island of Gran Canaria (Canary Islands, Spain): a genetic and clinical study. Hepatology 2000;32:1329-36. [Crossref] [PubMed]
- Frommer DJ. Urinary copper excretion and hepatic copper concentrations in liver disease. Digestion 1981;21:169-78. [Crossref] [PubMed]
- El Balkhi S, Poupon J, Trocello JM, et al. Determination of ultrafiltrable and exchangeable copper in plasma: stability and reference values in healthy subjects. Anal Bioanal Chem 2009;394:1477-84. [Crossref] [PubMed]
- El Balkhi S, Trocello JM, Poupon J, et al. Relative exchangeable copper: a new highly sensitive and highly specific biomarker for Wilson's disease diagnosis. Clin Chim Acta 2011;412:2254-60. [Crossref] [PubMed]
- Schmitt F, Podevin G, Poupon J, et al. Evolution of exchangeable copper and relative exchangeable copper through the course of Wilson's disease in the Long Evans Cinnamon rat. PLoS One 2013;8:e82323. [Crossref] [PubMed]
- Trocello JM, El Balkhi S, Woimant F, et al. Relative exchangeable copper: a promising tool for family screening in Wilson disease. Mov Disord 2014;29:558-62. [Crossref] [PubMed]
- Heissat S, Harel A, Um K, Brunet AS, et al. Evaluation of the accuracy of exchangeable copper and relative exchangeable copper (REC) in a mouse model of Wilson's disease. J Trace Elem Med Biol 2018;50:652-7. [Crossref] [PubMed]
- Guillaud O, Brunet AS, Mallet I, et al. Relative exchangeable copper: A valuable tool for the diagnosis of Wilson disease. Liver Int 2018;38:350-7. [Crossref] [PubMed]
- Lauwens S, Costas-Rodríguez M, Delanghe J, et al. Quantification and isotopic analysis of bulk and of exchangeable and ultrafiltrable serum copper in healthy and alcoholic cirrhosis subjects. Talanta 2018;189:332-8. [Crossref] [PubMed]
- Buckley WT, Vanderpool RA. Analytical variables affecting exchangeable copper determination in blood plasma. Biometals 2008;21:601-12. [Crossref] [PubMed]
- Venelinov TI, Beattie JH, Dainty JR, et al. Stable isotope pilot study of exchangeable copper kinetics in human blood plasma. J Trace Elem Med Biol 2007;21:138-40. [Crossref] [PubMed]
- Venelinov TI, Davies IM, Beattie JH. Dialysis-Chelex method for determination of exchangeable copper in human plasma. Anal Bioanal Chem 2004;379:777-80. [Crossref] [PubMed]
- Członkowska A, Tarnacka B, Möller JC, et al. Unified Wilson’s Disease Rating Scale - a proposal for the neurological scoring of Wilson’s disease patients. Neurol Neurochir Pol 2007;41:1-12. [PubMed]
- Siaj R, Sauer V, Stoppeler S, et al. Dietary copper triggers onset of fulminant hepatitis in the Long-Evans cinnamon rat model. World J. Gastroenterol 2012;18:5542-50. [Crossref] [PubMed]
- Cauza E, Maier-Dobersberger T, Polli C, et al. Screening for Wilson’s disease in patients with liver diseases by serum ceruloplasmin. J Hepatol 1997;27:358-62. [Crossref] [PubMed]


