
Positive correlation between telomere length and mitochondrial copy number in breast cancers
We read with great interest the article published by Campa et al. (1) about mitochondrial DNA (mtDNA) copy and telomere length (TL) in breast cancer. It suggested that telomere elongation and mitochondrial instability contribute to the carcinogenesis in breast cancer. TL and mtDNA copy number have been considered as important factor to cancer progression in various cancers. And their correlation in normal tissues and cancers also has been observed by several authors (2,3). However, Campa et al. (1) did not present the correlation between TL and mtDNA copy number in breast cancer. Recently, we are studying clinical and prognostic values of TL and mtDNA copy number in breast cancers, therefore, their association was analyzed.
TL and mtDNA copy were measured by real-time polymerase chain reaction (PCR) in 128 breast cancer. The study was approved by the Institutional Review Board at Dongsan Medical Center. We calculated the ratio of TL and mtDNA copy in tumors to that in paired normal tissues (3). And Pearson correlation coefficients were calculated to evaluate the relationships between TL and mtDNA copy.
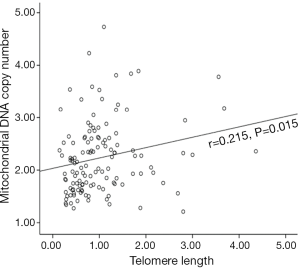
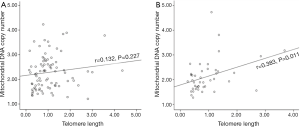
The average TL and mtDNA copy number in breast cancer were 1.02±0.75 and 2.23±0.69, respectively. Our result showed that TL was positively associated with mtDNA copy number in breast cancers (r=0.215, P=0.015) (Figure 1). It was in agreement with previous studies in healthy peoples and some cancers (2,3). When stratified by age (65 years old), a significant correlation was not identified in young patients with breast cancer (r=0.132, P=0.227) (Figure 2A); however, there was a correlation in older patients (r=0.383, P=0.011) (Figure 2B). This correlation has never been reported anywhere until now.
Previous studies demonstrated that telomere shortening induced mitochondrial dysfunction via p53 suggesting this axis may be important pathway for aging or carcinogenesis (4,5). Loss of this correlation in young patient with breast cancer may induce the deregulation of the telomere-mitochondria axis, and it contributes to breast carcinogenesis, although the mechanism underlying this process remains unclear. More investigations about the molecular mechanisms of telomere crosstalk with mtDNA are warranted. The biological functions of telomere and mtDNA are of new interest for breast cancer research.
Acknowledgements
Funding: This study was supported by grants of the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2014R1A6A3A04058057). Additional support was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korean Government (MSIP) (grant no. NRF-2016R1A5A2945889).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Campa D, Barrdahl M, Santoro A, et al. Mitochondrial DNA copy number variation, leukocyte telomere length, and breast cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Breast Cancer Res 2018;20:29. [Crossref] [PubMed]
- Kim JH, Kim HK, Ko JH, et al. The relationship between leukocyte mitochondrial DNA copy number and telomere length in community-dwelling elderly women. PLoS One 2013;8:e67227. [Crossref] [PubMed]
- Jung SJ, Cho JH, Park WJ, et al. Telomere length is correlated with mitochondrial DNA copy number in intestinal, but not diffuse, gastric cancer. Oncol Lett 2017;14:925-9. [Crossref] [PubMed]
- Sahin E, Colla S, Liesa M, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 2011;470:359-65. [Crossref] [PubMed]
- Hu J, Hwang SS, Liesa M, et al. Antitelomerase therapy provokes ALT and mitochondrial adaptive mechanisms in cancer. Cell 2012;148:651-63. [Crossref] [PubMed]


