
Clinical outcomes following long GnRHa ovarian stimulation with highly purified human menopausal gonadotropin plus rFSH or rFSH in patients undergoing in vitro fertilization-embryo transfer: a multi-center randomized controlled trial
Introduction
Highly purified human menopausal gonadotropin (HP-HMG) and recombinant human follicle-stimulating hormone (rFSH) have been widely used for ovarian stimulation in infertile women undergoing assisted reproductive technology (ART) (1). However, the impact of different gonadotropin preparations on women, who underwent controlled ovarian stimulation (COS) in in vitro fertilization-embryo transfer (IVF-ET), is still controversial. Two meta-analyses reported slightly higher live birth rate when using HMG for COS in comparison with rFSH in low-dose GnRH agonist long protocols (2,3).
Luteinizing hormone (LH) plays a role in follicular development and periovulatory, and it involves in ovulation induction, completion of meiosis I, early luteinization and progesterone production. Ovarian steroidogenesis can be driven by activation of a low number (around 1%) of LH receptors (4-6). In a meta-analysis, that including 40 randomized controlled trials, it found significantly more oocytes were retrieved and higher clinical pregnancy rates were observed with rFSH+rLH versus rFSH alone in poor responders (4). In a recent survey (7), the most common form of LH supplementation used in poor ovarian response (POR) was HMG+rFSH, followed by HMG alone, rLH+rFSH and low-dose HCG+rFSH. However, the use of LH supplementation during ovarian stimulation has long been a controversy, and there was study have reported the conflicting evidence (6). The objective of this study was to compare outcomes of HP-HMG+rFSH versus rFSH alone in patients undergoing IVF-ET treatment with antagonist protocol.
Methods
Study population
In this clinical trial, patients, who underwent IVF-ET treatment from 6 reproductive centers of China between May 2014 and December 2015, were recruited. This study complied with the Declaration of Helsinki and Good Clinical Practices (GCP) and was approved by the ethics committees of all participating centers. The study was registered at the Chinese Clinical Trial Registry on 21 April 2014 (http://www.chictr.org.cn/, Unique Identifier: ChiCTR-TRC-14004552).
The inclusion criteria were: (I) aged 20–37 years, BMI 18–24 kg/m2 and weight 40–80 kg, with regular menstrual cycle (21–35 days); (II) infertility (more than 1 year of free intercourses) with no history of IVF treatment; (III) basal FSH <10 U/L and LH <10 U/L; (IV) normal uterine anatomy confirmed by transvaginal ultrasound examination and in some cases hysterosalpingography and hysteroscopy; (V) no evidence of hydrosalpinx or ovarian cyst or endometrioma; (VI) antral follicle count (AFC) >6; and (VII) signed written informed consent. The exclusion criteria were: (I) had polycystic ovary syndrome, endometriosis of stage III/IV, hyperprolactinemia or other significant systemic disease (endocrine or metabolic abnormalities); (II) use of the following drugs within 1 month prior to randomization: clomiphene citrate, metformin, gonadotropin or GnRH analogues; (III) smokes >10 cigarettes per day within 3 months of recruitment; (IV) history of chemotherapy, radiotherapy, or ovarian surgery.
Study design
This study was a non-blinded, multi-center randomized clinical trial, it was designed to compare the therapeutic efficacy between HP-HMG (Menopur®, Ferring Pharmaceutical, Ltd., Copenhagen, Denmark) plus rFSH (Gonal-F®, Merck Serono, Geneva, Switzerland) and rFSH alone in GnRH agonist long protocols. An independent statistician provided sealed envelopes, containing two randomized groups (1:1 ratio) with a block size 4. The baseline serum estradiol (E2), FSH and LH levels, endometrial thickness and antral follicle diameter were confirmed strong down regulation after treatment with Triptorelin acetate (Decapeptyl®, Ferring Pharmaceutical, Ltd., Copenhagen, Denmark) for 14–20 days, then the randomization were performed. Based on the grouping stipulated inside the envelope, the patients were randomized into HP-HMG+rFSH group and rFSH group.
Sample size calculation
In this study, the P level on the day of HCG administration was as the primary objectives. Calculation of the sample size was based on two binomial proportions (logarithm of odds ratio); the significance level of the two-sided test was set at α=0.05, the power was 80%. Assuming a difference in P level between the 2 therapeutic regimens to be 0.5 nmol/L; the number of subjects needed in each group was 304 patients.
Treatment and monitor
Down regulation was achieved by using Triptorelin acetate (0.05 mg/day) 5–7 days before the onset of the next menstrual cycle. The initial dose of gonadotrophin used in HP-HMG+rFSH group was 75 IU HP-HMG + 75 IUrFSH for those weighted ≥60 kg and 75 IU HP-HMG + 150 IU rFSH for those weighted >60 kg, whereas the dose in rFSH group was 150 IU rFSH for those weighted ≤60 kg and 225 IU rFSH for those weighted >60 kg. After 5 days of continuous subcutaneous injection, the P and E2 levels in blood samples were detected. Then, according to the size of the follicle and the result of ovulation stimulation (7–15 follicles available for retrieval) (8), the dosage of rFSH was determined. If there were more than 4 follicles with diameter ≥16 mm or 3 follicles with diameter ≥18 mm (9), then 6500 IU HCG (Ovidrel®, MerckSerono, Geneva, Switzerland) was subcutaneously injected within 1 day to induce final follicular maturation, meanwhile the content of LH, E2 and P levels were measured by the central laboratory method. Oocytes were collected 36 h (±2 h) after administration of HCG. Endometrial thickness was measured on both days of initiation of gonadotrophin therapy and the HCG trigger day.
The morphology of cumulus oocyte was observed during oocyte retrieval operation, then after oocyte retrieved 3 h (±1 h) the assisted fertilization was carried out. After oocyte retrieved 20 h (±1 h), 44 h (±1 h) and 68 h (±1 h), the fertilization and embryo quality were evaluated by an experienced embryologist using an inverted microscope. Fresh embryo transfer was performed on 3 days after fertilization. It was worth noting that patients with ovarian hyper-stimulation syndrome (OHSS) risk or elevation of progesterone on the day of HCG trigger (>8 nmol/L) should be freeze the embryo for transfer in a subsequent cycle. Luteal support was started on the day of oocyte retrieval and performed through intramuscularly administering P (60 mg/day). After embryo transfer 14 days, the blood samples were obtained for HCG measurement to confirm whether pregnancy. If the pregnancy test was positive, ultrasound examination could be offered 2 weeks later to confirm the ability and location of pregnancy.
Outcome
Clinical pregnancy was defined as the presence of one or more gestational sacs detected through ultrasound scan 4–5 weeks after embryo transfer. Biochemical pregnancy was defined as plasma HCG of >10 IU/L 14 days after embryo transfer, with no subsequent evidence of any intrauterine gestational sac on ultrasonography. The primary outcomes were the P levels on the day of HCG injection. The secondary outcomes include the levels of E2, LH; follicle number and endometrial thickness on the day of HCG injection; the number of oocytes retrieved per cycle initiated; fertilization rate; cleavage rate; implantation rate; rate of moderate/severe OHSS; cycles with fresh embryo transfer and clinical pregnancy.
Statistical analysis
SPSS17.0 software was used for statistical analysis of data obtained in this study; the results were expressed as mean ± standard deviation (SD) or proportion; t-test and chi-square test were used for comparison between groups. P<0.05 was considered statistically significant.
Results
Baseline characteristics
In this study, a total of 955 women were assessed for eligibility. Finally, after administration of Triptorelin (0.05 mg/day) for down-regulation therapy, 610 patients were randomized into two groups: HP-HMG+rFSH group (n=305) received HP-HMG+rFSH and rFSH group (n=305) received rFSH (Figure 1). Baseline demographic characteristics and hormone level at the beginning of ovulation induction therapy were presented in Table 1, there was no statistically significant differences between two groups (all P>0.05).

Full table
Treatment outcome
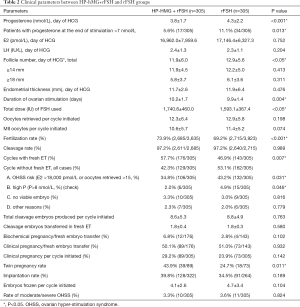
The clinical outcomes of the two groups were compared in Table 2. In the rFSH group, the P level on the day of HCG trigger was significantly higher than HP-hMG+rFSH group (4.3±2.2 vs. 3.8±1.7 nmol/L, P<0.001). The percentage of patients who with progesterone at the end of stimulation >7 nmol/L was also higher in the rFSH group, compared with HP-hMG+rFSH group (11.1% vs. 5.6%, P=0.013). The number of oocytes retrieved in rFSH group was significantly higher than HP-hMG+rFSH group (12.9±5.6 vs. 11.9±6.0, P<0.05). On the contrary, the fertilization rate in the rFSH group was significantly lower than HP-hMG+rFSH group (69.2% vs. 73.9%, P<0.001). Simultaneously, the percentage of cycles with fresh embryo transfer in rFSH group was significantly lower than HP-hMG+rFSH group (49.6% vs. 57.5%, P=0.007). However, there was no significant difference in the implantation and clinical pregnancy rate between the two groups.
Full table
Discussion
Many studies have reported that the high P level on the day of HCG administration on endometrial receptivity may reduce implantation rate and pregnancy rate (10-13). In this study, we found that there was no difference of elevated P level on day of HCG on implantation rate between two groups. There were two possible explanations as following. Firstly, in our study, based on the recommendation of earlier reports, we have frozen all embryos for later transfer in women with high P level on the day of HCG administration. Such a policy would avoid the replacement of (fresh) embryos in cycles with a lower chance of successful consequent upon the high progesterone. Secondly, the number of fresh embryo cycles in our study was relatively small (176 vs. 143).
Recently, self-injecting pen type of rFSH has been widely used and it has been shown to improve the patient’s convenience (14). In comparison with daily administration of short-acting GnRHa, a single administration of long-acting GnRHa can append these advantages by reducing the number of injections in controlled ovarian hyperstimulation (COH) (15). In the long protocol, the combination of long-acting GnRHa with self-injecting pen type rFSH can reduce the number and the cost of hospital visits to have an injection of GnRHa and gonadotrophins. Taken together, a single administration of long-acting GnRHa in combination with self-injecting pen type of rFSH can significantly improve the patient’s convenience and comfort. In women receiving rFSH alone, the number of follicles retrieved was significantly higher than rFSH+HP-HMG group, which was consistent with previous studies (16). One possible explanation for the greater number of oocytes retrieved in the rFSH group was the greater potency of rFSH compared HMG. Furthermore, several previous reports have suggested that 75 units of HMG were equivalent to 56 units FSH activity (17). Nevertheless, the increasing number of oocytes in rFSH group did not translate into an increasing number of embryos produced. On the contrary, the number of embryos produced in the two groups was similar, because the higher number of oocytes retrieved in the rFSH group was offset by a lower fertilization rate. The above findings were consistent with a previous report, that the LH supplementation might reduce the number of oocyte retrieved, while improve quality of oocyte (18).
OHSS is one of the severe, occasionally lethal iatrogenic conditions of IVF in COH process (19). Besides the relationship with the individual difference, the incidence of OHSS is mainly correlated with multiple-follicle development and high E2 level (9). In our study, we found that the proportion of cases considered as high risk for development of OHSS in the rFSH group was significantly higher than that of the rFSH+HP-HMG group. Previous study reported that the addition of LH to ovarian stimulation protocol in IVF could reduce the OHSS risk (20), due to LH was able to reduce the recruitment of small follicles in early follicular phase (21), increase the development of large follicles in late follicular phase, reduce the number of oocytes retrieved, and thus decrease the incidence of OHSS. In our study, some measures including freezing all embryos for later replacement and dextran infusion were taken to reduce the occurrence of OHSS in women considered as high risk. In this study, the rate of moderate/severe OHSS in the rFSH group was similar to the rFSH + HP-HMG group.
Several controversies exist in relation to the role of LH supplementation during ovarian stimulation. Previously, some studies compared rLH+rFSH with rFSH alone (22), some compared HMG to rFSH alone (23), and some compared HCG+rFSH with rFSH alone (24), all showed inconsistent results. A systematic review and meta-analysis has demonstrated that rLH supplementation did not increase ongoing pregnancy rate, however it could reduce the amount of rFSH required and the oestradiol level was higher on the day of HCG administration in the LH supplemented group than the rFSH alone group (25). Another recent systematic review and meta-analysis found the more significant oocytes were retrieved and the higher clinical pregnancy rates were observed with rFSH plus rLH versus rFSH alone in poor responders (4), which seem to support that LH supplementation is beneficial to PORs. Furthermore, the role of LH supplementation in normal responders, who underwent long GnRHa protocol for IVF-ET, remains controversial. Some investigators reported that LH supplementation could increase the pregnancy rate and reduce OHSS rate (26), but other investigators found that the addition of LH to FSH did not improve clinical outcomes in normal responders (27). There were several reasons for the different observations. Firstly, the dose of LH used in the various studies was not standardized. Secondly, the ratio of LH to FSH appeared to have significant impact on the outcome, and some investigators have reported that the optimal ratio of LH to FSH was around 1:2 (28), which was also taken in our study.
Conclusions
In conclusion, the use of combined HP-HMG with FSH may be superior to rFSH alone in stimulating the ovary in normal responders undergoing IVF treatment. Furthermore, the further prospective studies with large sample are still needed to confirm the study.
Acknowledgements
The authors thank Hong-Gang Yi for statistical analyses. We also thank all doctors and nurses of the participating centers.
Funding: The study was supported by projects from the National 973 program (2012CB944902), Research fund of National Health and Family Planning Commission of China (201402004, 201002013), and the Jiangsu Province Special Program of Medical Science (BL2012009, ZX201110, FXK201221).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study complied with the Declaration of Helsinki and Good Clinical Practices (GCP) and was approved by the ethics committees of all participating centers.
References
- Ye H, Huang G, Pei L, et al. Outcome of in vitro fertilization following stimulation with highly purified hMG or recombinant FSH in downregulated women of advanced reproductive age: a prospective, randomized and controlled trial. Gynecol Endocrinol 2012;28:540-4. [Crossref] [PubMed]
- Hompes PG, Broekmans FJ, Hoozemans DA, et al. Effectiveness of highly purified human menopausal gonadotropin vs. recombinant follicle-stimulating hormone in first-cycle in vitro fertilization-intracytoplasmic sperm injection patients. Fertil Steril 2008;89:1685-93. [Crossref] [PubMed]
- Coomarasamy A, Afnan M, Cheema D, et al. Urinary hMG versus recombinant FSH for controlled ovarian hyperstimulation following an agonist long down-regulation protocol in IVF or ICSI treatment: a systematic review and meta-analysis. Hum Reprod 2008;23:310-5. [Crossref] [PubMed]
- Lehert P, Kolibianakis EM, Venetis CA, et al. Recombinant human follicle-stimulating hormone (r-hFSH) plus recombinant luteinizing hormone versus r-hFSH alone for ovarian stimulation during assisted reproductive technology: systematic review and meta-analysis. Reprod Biol Endocrinol 2014;12:17. [Crossref] [PubMed]
- Sen A, Caiazza F.. Oocyte maturation: a story of arrest and release. Front Biosci (Schol Ed) 2013;5:451-77. [Crossref] [PubMed]
- Fischer R.. Understanding the role of LH: myths and facts. Reprod Biomed Online 2007;15:468-77. [Crossref] [PubMed]
- Patrizio P, Vaiarelli A, Levi Setti PE, et al. How to define, diagnose and treat poor responders? Responses from a worldwide survey of IVF clinics. Reprod Biomed Online 2015;30:581-92. [Crossref] [PubMed]
- Arce JC, Nyboe Andersen A, Collins J. Resolving methodological and clinical issues in the design of efficacy trials in assisted reproductive technologies: a mini-review. Hum Reprod 2005;20:1757-71. [Crossref] [PubMed]
- Chen Y, Ye B, Yang X, et al. Predicting the outcome of different protocols of in vitro fertilization with anti-Muullerian hormone levels in patients with polycystic ovary syndrome. J Int Med Res 2017;45:1138-47. [Crossref] [PubMed]
- Hill MJ, Royster GD, Healy MW, et al. Are good patient and embryo characteristics protective against the negative effect of elevated progesterone level on the day of oocyte maturation? Fertil Steril 2015;103:1477-84.e1-5.
- Huang R, Fang C, Xu S, et al. Premature progesterone rise negatively correlated with live birth rate in IVF cycles with GnRH agonist: an analysis of 2,566 cycles. Fertil Steril 2012;98:664-70.e2. [Crossref] [PubMed]
- Kolibianakis EM, Venetis CA, Bontis J, et al. Significantly lower pregnancy rates in the presence of progesterone elevation in patients treated with GnRH antagonists and gonadotrophins: a systematic review and meta-analysis. Curr Pharm Biotechnol 2012;13:464-70. [Crossref] [PubMed]
- Orvieto R, Nahum R, Meltzer S, et al. GnRH agonist versus GnRH antagonist in ovarian stimulation: the role of elevated peak serum progesterone levels. Gynecol Endocrinol 2013;29:843-5. [Crossref] [PubMed]
- Cheon KW, Song SJ, Choi BC, et al. Comparison of clinical efficacy between a single administration of long-acting gonadotrophin-releasing hormone agonist (GnRHa) and daily administrations of short-acting GnRHa in in vitro fertilization-embryo transfer cycles. J Korean Med Sci 2008;23:662-6. [Crossref] [PubMed]
- Pang SC. A pen injection device for self-administration of recombinant follicle-stimulating hormone for fertility treatments. Expert Rev Med Devices 2005;2:27-32. [Crossref] [PubMed]
- Platteau P, Smitz J, Albano C, et al. Exogenous luteinizing hormone activity may influence the treatment outcome in in vitro fertilization but not in intracytoplasmic sperm injection cycles. Fertil Steril 2004;81:1401-4. [Crossref] [PubMed]
- Ema M, Harazono A, Miyawaki E, et al. The Human Menopausal Gonadotrophin Reference Standard (Control 961) of the National Institute of Health Sciences. Eisei Shikenjo Hokoku 1996.138-40. [PubMed]
- Lisi F, Rinaldi L, Fishel S, et al. Evaluation of two doses of recombinant luteinizing hormone supplementation in an unselected group of women undergoing follicular stimulation for in vitro fertilization. Fertil Steril 2005;83:309-15. [Crossref] [PubMed]
- Varnagy A, Bodis J, Manfai Z, et al. Low-dose aspirin therapy to prevent ovarian hyperstimulation syndrome. Fertil Steril 2010;93:2281-4. [Crossref] [PubMed]
- Figen Turkcapar A, Seckin B, Onalan G, et al. Human Menopausal Gonadotropin versus Recombinant FSH in Polycystic Ovary Syndrome Patients Undergoing In Vitro Fertilization. Int J Fertil Steril 2013;6:238-43. [PubMed]
- Filicori M, Cognigni GE, Taraborrelli S, et al. Luteinzing hormone activity in menotropins optimizes folliculogenesis and treatment in controlled ovarian stimulation. J Clin Endocrinol Metab 2001;86:337-43. [Crossref] [PubMed]
- Musters AM, van Wely M, Mastenbroek S, et al. The effect of recombinant LH on embryo quality: a randomized controlled trial in women with poor ovarian reserve. Hum Reprod 2012;27:244-50. [Crossref] [PubMed]
- De Placido G, Mollo A, Alviggi C, et al. Rescue of IVF cycles by HMG in pituitary down-regulated normogonadotrophic young women characterized by a poor initial response to recombinant FSH. Hum Reprod 2001;16:1875-9. [Crossref] [PubMed]
- Ferrari B, Barusi L, Coppola F. Clinical and endocrine effects of ovulation induction with FSH and hCG supplementation in low responders in the midfollicular phase. A pilot study. J Reprod Med 2002;47:137-43. [PubMed]
- Fan W, Li S, Chen Q, et al. Recombinant Luteinizing Hormone supplementation in poor responders undergoing IVF: a systematic review and meta-analysis. Gynecol Endocrinol 2013;29:278-84. [Crossref] [PubMed]
- Caserta D, Lisi F, Marci R, et al. Does supplementation with recombinant luteinizing hormone prevent ovarian hyperstimulation syndrome in down regulated patients undergoing recombinant follicle stimulating hormone multiple follicular stimulation for IVF/ET and reduces cancellation rate for high risk of hyperstimulation? Gynecol Endocrinol 2011;27:862-6. [Crossref] [PubMed]
- Kovacs P, Kovats T, Kaali SG. Results with early follicular phase recombinant luteinizing hormone supplementation during stimulation for in vitro fertilization. Fertil Steril 2010;93:475-9. [Crossref] [PubMed]
- Fabregues F, Creus M, Casals G, et al. Outcome from consecutive ICSI cycles in patients treated with recombinant human LH and those supplemented with urinary hCG-based LH activity during controlled ovarian stimulation in the long GnRH-agonist protocol. Gynecol Endocrinol 2013;29:430-5. [Crossref] [PubMed]


