
High-glucose treatment regulates biological functions of human umbilical vein endothelial cells via Sirt1/FOXO3 pathway
Introduction
Angiogenesis is characterized by the formation of new capillaries that sprout from pre-existing microvasculature. Pathological formation of blood vessels is involved in the pathogenesis of several ophthalmological diseases including diabetic retinopathy (DR). In developed countries, DR has become a major cause of blindness (1). Available studies have shown that diabetic vascular dysfunction is responsible for the retinopathy and blindness in diabetic patients. For endothelial cells that undergo hyperglycaemia-induced angiogenesis, their cellular functions (such as proliferation, migration, cell senescence and apoptosis) are significantly altered (2).
The silent information regulator 2 (Sir2) family of enzymes is a group of highly conserved nicotinamide adenine dinucleotide (NAD+)-dependent histone/non-histone deacetylases that exist widely among a range of organisms from archaebacteria to mammals. Sir2 is involved in the regulation of energy restriction-induced longevity and thus Sir2 is also known as the “longevity gene” (3). To date, genes homologous to Sir2 have been found in most species and are collectively known as the Sirtuin (SIRT) family. Sirt1 was the first member of Sirtuin family identified in mammals and has become the most extensively studied protein in Sirtuin family. Sirt1 is widely expressed in human body, including the retina and various types of endothelial cells [such as human umbilical vein endothelial cells (HUVECs) and human microvascular endothelial cells]. Sirt1 has complex physiological functions. Through deacetylating substrates, Sirt1 participates in the regulation of DNA damage repair, oxidative stress, inflammation, cell metabolism, cell senescence, apoptosis and abnormal proliferation (4). A variety of studies have reported that Sirt1 plays an important role in the development and progression of vascular diseases (5). Sirt1 is a key protein in endothelial cells and can control postnatal vascular growth. During the angiogenesis, Sirt1 is highly expressed in the vasculature to regulate angiogenesis. Down-regulation of Sirt1 expression may reduce the expression of genes related to vascular development and remodeling, and inhibit the budding and branching of endothelial cells during angiogenesis (6). Sirt1 mainly functions through its downstream transcription factors [such as forkhead box O (FOXO)]. FOXO3 is an important transcription factor of FOXO family and plays a crucial role in vascular homeostasis (7).
The present study aimed to explore the role of Sirt1/FOXO3 pathway in the effects of high glucose on HUVECs in vitro.
Methods
Cell culture and grouping
HUVECs were purchased from the Cell Bank in the Shanghai Institute for Biological Sciences of the Chinese Academy of Sciences and maintained in RPMI-1640 medium (GIBCO) supplemented with 10% foetal calf serum (FCS; GIBCO, Australia), 100 U/mL streptomycin and 100 U/mL penicillin (GIBCO) in a humidified atmosphere with 5% CO2 at 37 °C (8). HUVECs of passage 4–10 were used in the following experiments. Human embryonic kidney 293T cells (HEK293FT) were purchased from the American Type Culture Collection (ATCC) and grown in Dulbecco’s modified Eagle’s medium (DMEM; GIBCO, Australia) supplemented with 10% FCS, 100 U/mL streptomycin and 100 U/mL penicillin. Cells were randomly divided into normal control group (5 mM glucose), high glucose group (30 mM glucose), 30 mM glucose + shsirt1 group (30 mM + shsirt1), 30 mM glucose + Sirt1 over-expression group (30 mM + Sirt1), 30 mM glucose + Sirt1 agonist SRT group (30 mM + SRT), 30 mM glucose + SRT + FOXO3 silencing group (30 mM + SRT + siFOXO3).
Plasmids, transfection and virus generation
The wild-type human Sirt1 vector was constructed. The deletion human Sirt1 construct was made using the restriction enzymes Age I and E.col I (NEB) and PCR. The shRNAs targeting scramble and Sirt1 were synthesized in JieRui BioTech Company and are as follows: scramble, CCTAAGGTTAAGTCGCCCTCG; Sirt1, CCGGCAGGTCAAGGGATGGTATTTACTCGAGTAAATACCATCCCTTGACCTGTTTTTG and AATTCAAAAACAGGTCAAGGGATGGTATTTACTCGAGTA AATACCATCCCTTGACCTG. The annealing shRNA constructs were cloned into pLKO.1 lentiviral vector via the AgeI and EcoRI restriction sites. HEK293FT cells were transfected with X-treme GENE HP (Roche) reagent. Lentivirus particles containing the helping plasmid and the target gene plasmid were generated by co-transfection in HEK293FT cells. The virus was then harvested 2 days after transfection. HEK293FT cells (1.2×105 cells/well, 6-well plate) at 80–90% confluence was used for transfection.
RT-PCR and Real-time PCR
Total RNA was isolated from HUVECs using TRIzol reagent (Invitrogen), and 500 ng of RNA from each sample was reverse-transcribed (RT) into cDNA using the PrimeScript™ RT reagent kit (TaKaRa). The cDNA was used as the template for real-time PCR. The real-time PCR primers were synthesized in the GenRey Company.
Antibodies and Western blotting
Mouse anti-human Sirt1 (1F3) mAb (Cell Signaling Technology, #8469, 1:1,000), rabbit anti-human FOXO3a (D19A7) mAb (Cell Signaling Technology, #12829, 1:1,000), PGC1-α (Abcam, ab54481, 1:1,000), and GAPDH (Santa Cruz Biotechnology, sc-47724, 1:1,000) antibodies were used for Western blotting. In brief, HUVECs were lysed with radio-immunoprecipitation assay (RIPA) buffer [0.1% sodium dodecyl sulphate (SDS), 1.0% Igepal CA-630, 0.5% sodium deoxycholate, 50 mM Tris and 150 mM NaCl, pH 8.0; Shanghai Sangong Company] on ice for 20 min. The lysates were centrifuged at 12,000 ×g for 10 min at 4 °C, and the protein content was determined by bicinchoninic assay (BCA). Equal amounts of protein were loaded and then subjected to SDS-PAGE, and visualization was done with Clarity Western ECL Substrate (Bio-Rad, 1705061). The optical density of each protein band was measured and compared to that of internal reference.
DAPI staining
After three 5-minute washes in phosphate-buffered saline (PBS), cells were incubated with 100 nmol/L Hochest-33342 (BiYuntian) for 5 min at room temperature in dark. Then, cells were washed thrice in PBS (5 min for each) and then observed under a fluorescence microscope (Nikko). Cells were photographed, and images were analyzed using ImageJ software. Cells were counted in at least 5 randomly selected fields for each sample.
Flow cytometry of cell apoptosis
Single cell suspension was obtained after digestion with trypsin-EDTA according to the manufacturer’s instructions (KeyGEN, KGA105 or KGA511). Then, cells were re-suspended in PBS and stained with FITC-conjugated annexin V and propidium iodide (PI) with apoptosis detection kit (KeyGEN, KGA105 or KGA511). Flow cytometry (FACSCalibur; BD Biosciences) was performed at 488 nm with standard emission filter. The baseline fluorescence noise was established with untreated cells.
Cell proliferation detection
HUVECs were seeded into a 96-well plate (500 cells/well), followed by incubation for 24 h. Then, the medium was removed, and cells were rinsed thrice with PBS and maintained overnight in serum-free RPMI-1640 medium (GIBCO, Australia) containing 100 U/mL penicillin and 100 mg/mL streptomycin. After addition of 10 µL of MTT solution, incubation was done at 37 °C for 4 h. The medium was removed, and 150 µL of DMSO (Sigma) was added to each well. After incubation for 10 min at 37 °C at constant shaking, the absorbance was detected at 490 nm. Experiments were conducted in duplicate for each group.
Cell migration assay
Scratch wound healing assay
Cells were seeded into 6-well plates (3×105 cells/well). Once cell confluence reached 90%, cells were maintained in serum-free medium for 24 h for cell cycle synchronization. The monolayer cells were scratched in a straight line across the centre of the plate bottom using a 200-µL pipette tip, leaving a cell-free gap approximately 0.8 mm in width. The detached cells were removed by washing with serum-free medium. Five marks were made along the edge of each scratch line with the same interval for following measurements. Measurement was done at above 5 marks with ImageJ software, and the mean was calculated. Cells in different groups were maintained for 0, 24 and 48 h at 37 °C in a humidified environment with 5% CO2 and then observed under an inverted microscope (105 magnification). The area between the two edges of the scratch was measured at various time points using ImageJ software. The HUVEC migration rate at 0, 24 and 48 h were calculated as follow: migration rate = (area at 0 h − area at a specific time point)/area at 0 h. The migration rate reflected the migration ability of these cells.
Transwell migration assay
Cell migration was examined by Transwell assay with PVPF membranes (pore size: 0.8 µm). Cells in different groups were washed twice with serum-free RPMI-1640 medium. Then, cells were counted and re-suspended in RPMI-1640 medium containing 1% fetal bovine serum (FBS) at 4×104 cells/100 µL. These cells were added to the upper chambers of the Transwell plates, while 600 µL of RPMI-1640 containing 10% FBS was added into the lower chambers. After incubation at 37 °C in an atmosphere with 5% CO2 for 10 h, the upper chambers were collected and cells on the upper surfaces of upper chambers were removed with a cotton swab. Cells on the lower surfaces of upper chambers were fixed in formaldehyde at room temperature for 20 min, and then stained with Hochest33342 in dark for 5 min. After washing more than 3 times with 1× PBS, cells were examined and counted under a microscope.
Detection of FOXO3 activity
All reagents in the FOXO3 Activity Detection Kit were kept at room temperature (25–28 °C) for 30 min before use. Samples were added to the plates (100 µL/well), followed by incubation at 37 °C for 2 h. Subsequently, the solution was removed, and biotin-conjugated antibody was added to the plates (100 µL/well). The plates were incubated at 37 °C for 1 h, and the solution was removed. After washing thrice with wash solution (200 µL/well; 3 min for each), the plates were air-dried. The horseradish peroxidase working solution was added to the plates (100 µL/well), followed by incubation at 37 °C for 1 h. The solution was then removed, and the plates were washed 6 times with wash solution (200 µL/well, 3 min for each) and then air-dried. The substrate reaction buffer (90 µL/well) was added to each well, followed by incubation at 37 °C in dark for 30 min. Then, 50 µL of stop solution was added to each well to terminate the reaction. The optical density was measured at 450 nm within 5 min using a microplate reader.
Statistical analysis
All data are represented as mean ± standard error (SEM). Two-tailed Student’s t-test (for parametric data) or Mann-Whitney test (for nonparametric data) was used for comparisons depending on their distribution status. A value of P less than 0.05 was considered statistically significant. Prism 5 (GraphPad) was used for statistical analysis.
Results
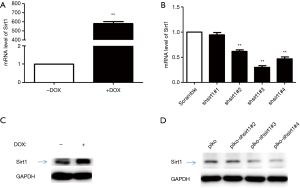
Construction of Sirt1 over-expressing HUVECs
Stable Sirt1 over-expressing HUVECs and stable Sirt1 silenced HUVECs were constructed. Both cell lines were subjected to Western blotting and quantitative PCR to validate the expression of Sirt1 (Figure 1). Results showed Sirt1 expression increased significantly in Sirt1 over-expressing HUVECs and reduced markedly in Sirt1 silenced HUVECs.
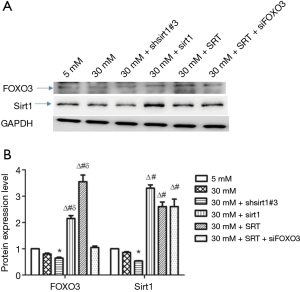
Protein expression of Sirt1 and FOXO3
Western blotting showed that the protein expression of Sirt1 and FOXO3 decreased in high-glucose (30 mM glucose) group compared with normal control group (5 mM glucose) although there was no significant difference. Under high-glucose conditions, silencing of Sirt1 expression reduced the protein expression of Sirt1 and FOXO3, whereas Sirt1 over-expression or Sirt1 agonist (SRT) increased the protein expression of Sirt1 and FOXO3. In addition, inhibition of FOXO3 expression and administration of Sirt1 agonist under high-glucose conditions increased Sirt1 expression and decreased FOXO3 expression in HUVECs (Figure 2).
Detection of cell proliferation
As shown in Figure 3A, Sirt1 over-expression significantly promoted HUVEC proliferation (P<0.05 vs. un-transfected cells). In contrast, silencing of Sirt1 expression markedly inhibited HUVEC proliferation (P<0.05 vs. un-transfected cells) (Figure 3B).
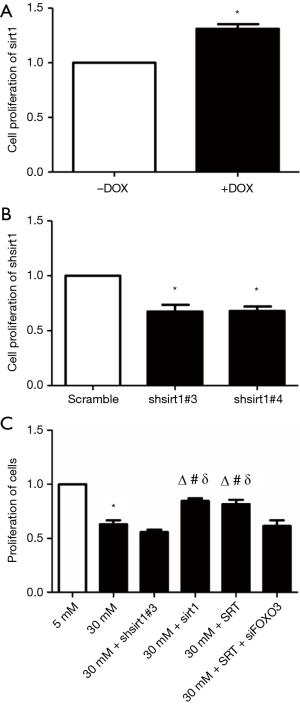
Compared with normal control group (5 mM glucose), high-glucose (30 mM glucose) significantly inhibited HUVEC proliferation (P<0.01). Inhibition of Sirt1 expression under high-glucose conditions (30 mM glucose + shsirt1) also markedly suppressed HUVEC proliferation. No significant difference was observed in the proliferation between 30 mM glucose + shsirt1 group and 30 mM glucose group (P>0.05). Under high-glucose conditions, Sirt1 over-expression (30 mM glucose + Sirt1) or Sirt1 agonist SRT (30 mM glucose + SRT) increased HUVEC proliferation. Compared with 30 mM glucose group and 30 mM glucose + shsirt1 group, the cell proliferation significantly increased in 30 mM glucose + Sirt1 group and 30 mM glucose + SRT group (P<0.01). Inhibition of FOXO3 expression and administration of Sirt1 agonist SRT under high-glucose conditions (30 mM glucose + SRT + siFOXO3) significantly suppressed HUVEC proliferation (P<0.05 vs. 30 mM glucose + Sirt1 group and 30 mM glucose + SRT group). However, the proliferation in 30 mM glucose + SRT + siFOXO3 group was similar to that in high-glucose (30 mM) group and 30 mM glucose + shsirt1#3 group (P>0.05). In contrast, the proliferation in 30 mM glucose + SRT + siFOXO3 group was significantly different from that in 30 mM glucose + Sirt1 group and 30 mM glucose + SRT group (P<0.01) (Figure 3C).
Scratch wound healing assay
Compared with normal control group (5 mM glucose group), high-glucose (30 mM) significantly inhibited HUVEC migration (P<0.01). Inhibition of Sirt1 expression under high-glucose conditions (30 mM glucose + shsirt1) also suppressed HUVEC migration. There was no significant difference in the migration between 30 mM glucose + shsirt1 group and high glucose (30 mM) group (P>0.05). Under high-glucose conditions, Sirt1 over-expression (30 mM glucose + Sirt1) or Sirt1 agonist SRT (30 mM glucose + SRT) markedly enhanced HUVEC migration. There was significant difference in the HUVEC migration between high glucose (30 mM) group and 30 mM glucose + shsirt1 group (P<0.01). Inhibition of FOXO3 expression and administration of Sirt1 agonist SRT under high-glucose condition (30 mM glucose + SRT + siFOXO3) markedly suppressed HUVEC migration. However, the HUVEC migration in 30 mM glucose + SRT + siFOXO3 group was similar to that in high-glucose (30 mM) group and 30 mM glucose + shsirt1 group (P>0.05). In contrast, the HUVEC migration in 30 mM glucose + SRT + siFOXO3 group was significantly different from that in 30 mM glucose + Sirt1 group and 30 mM glucose + SRT group (P<0.01) (Figure 4A,B).
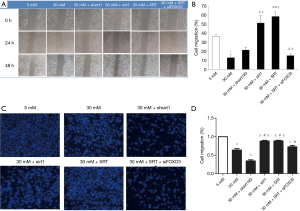
Transwell migration assay
Compared with normal control group (5 mM glucose), high-glucose conditions (30 mM glucose) significantly suppressed HUVEC migration (P<0.01). Inhibition of Sirt1 expression under high-glucose conditions (30 mM glucose + shsirt1) resulted in greater inhibition of HUVEC migration. A significant difference was observed in the migration capacity between 30 mM glucose + shsirt1 group and 30 mM glucose group (P<0.01). Under high-glucose conditions, Sirt1 over-expression (30 mM glucose + Sirt1) or Sirt1 agonist SRT (30 mM glucose + SRT) significantly enhanced HUVEC migration. In addition, there was significant difference between high-glucose (30 mM glucose) group and 30 mM glucose + shsirt1 group (P<0.01). Inhibition of FOXO3 expression and administration of Sirt1 agonist SRT under high-glucose conditions (30 mM glucose + SRT + siFOXO3) dramatically suppressed HUVEC migration. The migration rate in 30 mM glucose + SRT + siFOXO3 group was significantly different from that in high-glucose (30 mM) group and 30 mM glucose + SRT group (P<0.01). In contrast, the migration rate significantly increased in 30 mM glucose + SRT + siFOXO3 group compared with high glucose (30 mM) group (P<0.05) and 30 mM glucose + shsirt1 group (P<0.01) (Figure 4C,D).
Flow cytometry of cell apoptosis
Compared with normal control group (5 mM glucose), the apoptotic rate significantly increased in high-glucose (30 mM glucose) group (P<0.01). Silencing of Sirt1 expression under high-glucose conditions (30 mM glucose + shsirt1) further elevated apoptosis in HUVECs. The apoptotic rate was significantly elevated in 30 mM glucose + shsirt1 group compared with high-glucose (30 mM glucose) group (P<0.01). Under high-glucose conditions, Sirt1 over-expression (30 mM glucose + Sirt1) or Sirt1 agonist SRT (30 mM glucose + SRT) significantly reduced HUVEC apoptosis. There was marked difference in the apoptotic rate between high-glucose (30 mM glucose) group and 30 mM glucose + shsirt1 group) (P<0.01). Moreover, inhibition of FOXO3 expression and administration of Sirt1 agonist SRT under high-glucose conditions (30 mM glucose + SRT + siFOXO3) significantly enhanced apoptotic rate in HUVECs. The apoptosis rate in 30 mM glucose + SRT + siFOXO3 group was significantly different from that in high-glucose (30 mM glucose) group (P<0.01). In contrast, there was no significant difference in the apoptotic rate between 30 mM glucose + SRT + siFOXO3 group and 30 mM glucose + shsirt1 group (P>0.05). Significant difference in the apoptotic rate was observed between 30 mM glucose + SRT + siFOXO3 group and 30 mM glucose + Sirt1 group (P<0.01) as well as between 30 mM glucose + SRT + siFOXO3 group and 30 mM glucose + SRT group (P<0.01) (Figure 5).

Detection of PGC-1α expression
To further investigate whether above effects of Sirt1/FOXO3 pathway is related to PGC-1α, the expression of PGC-1α was detected (9). Results showed that the PGC-1α expression significantly elevated in high-glucose (30 mM glucose) group compared with normal control (5 mM glucose) group. Compared with normal control group, the PGC-1α expression remained elevated when Sirt1 expression was inhibited under high-glucose conditions (30 mM glucose + shsirt1 group). However, compared with high-glucose (30 mM glucose) group, the PGC-1α expression was down-regulated in 30 mM glucose + shsirt1 group. Under high-glucose conditions, Sirt1 over-expression (30 mM glucose + Sirt1) alone, or Sirt1 over-expression plus FOXO3 inhibition or Sirt1 agonist plus PGC-1α inhibition (30 mM glucose + SRT + siPGC-1α) resulted in the down-regulation of PGC-1α expression in HUVECs (Figure 6A).
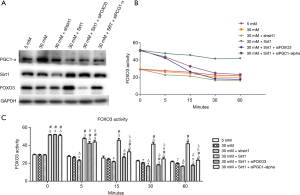
Detection of FOXO3 activity
The FOXO3 activity was further detected in HUVECs. Compared with normal control group (5 mM glucose), FOXO3 activity decreased slightly over time in high-glucose group. Silencing of Sirt1 expression under high-glucose conditions (30 mM glucose + shsirt1) further suppressed FOXO3 activity in HUVECs. Under high-glucose conditions, Sirt1 over-expression (30 mM glucose + Sirt1 group) significantly enhanced the FOXO3 activity in HUVECs. However, Sirt1 over-expression and FOXO3 inhibition under high-glucose conditions (30 mM glucose + Sirt1 + siFOXO3 group) markedly reduced FOXO3 activity in HUVECs. In contrast, Sirt1 over-expression plus PGC-1α inhibition (30 mM glucose + Sirt1 + siPGC-1α group) under high-glucose conditions slightly reduced FOXO3 activity in HUVECs (Figure 6B,C).
Discussion
Sirt1 is an NAD+-dependent deacetylase that acts on histones and a variety of transcription factors. As a multifunctional protein, Sirt1 is involved in a variety of cellular processes including differentiation, senescence, apoptosis, physiological rhythms, metabolic regulation, oxidative stress responses and others. In addition, Sirt1 is also an important transcriptional factor and a key regulator in some cellular signaling pathways (3,10,11). Sirt1 is highly sensitive to cellular redox and nutritional status. Studies have shown that SIRT1 is able to regulate gene stability and cellular metabolism, exerting protective effects on cardiomyocytes, islet beta cells and nerve cells. Strategies targeting Srit1 have been successfully employed for the treatment of some heart diseases, type 2 diabetes mellitus (T2DM) and neurodegenerative diseases. In clinical studies, Srit1 is also used for the treatment of T2DM (12,13). In recent years, several reports have highlighted Sirt1 as a key regulator of endothelial homeostasis. Sirt1 is abundantly expressed in the blood vessels and involved in the development and progression of various vascular diseases (6,14). Sirt1 may prevent functional decompensation of endothelial cells (15). By up-regulating Sirt1, resveratrol (a Sirt1 activator) may inhibit oxidative stress and inflammatory responses in vascular endothelial cells, protecting them from apoptosis (16). Studies have examined the role of Sirt1 and its downstream transcription factors in DR. Kowluru et al. found hyperglycaemia was able to reduce Sirt1 activity and p65 activation in the retina, which further activated the matrix metallopeptidase 9 (MMP-9) signaling pathway and promoted apoptosis. The Sirt1 agonist resveratrol is able to inhibit p65 acetylation and suppress p65-induced activation of MMP-9 signaling pathway, thereby reducing mitochondrial damage and apoptosis (17). Mortuza et al. found the Sirt1 expression significantly reduced in human retinal microvascular endothelial cells under high-glucose conditions, and induction of Sirt1 expression was found to attenuate the apoptosis of human retinal microvascular endothelial cells, thereby alleviating the microcirculatory disturbances in DR (18). Kubota et al. revealed that DM caused retinal inflammation by down-regulating AMP-activated protein kinase (AMPK) signaling pathway, and resveratrol was capable of reversing DM-induced AMPK dephosphorylation. In addition, resveratrol significantly reverses the inactivation of Sirt1 and phosphorylation of nuclear factor kappa-light-chain-enhancer in activated B cells (NF-κB), which reduces DM-induced retinal leukocyte adhesion and decreases the expression of intercellular adhesion molecule-1 (ICAM-1) and vascular endothelial growth factor (VEGF) in the retina, thereby inhibiting the inflammation (19). Sirt1 also plays an important regulatory role in ischemia- and hypoxia-induced vascular regeneration in late-stage DR. In a mouse model of oxygen-induced proliferative retinopathy, Chen et al. revealed up-regulation of Sirt1 expression in the retinal neurons of ischemic regions was able to regulate the stability of hypoxia-inducible factor (HIF)-1α and HIF-2α through deacetylation, which could regulate the expression of angiogenic factors and induce normal blood vessel development in the avascular regions. In contrast, Sirt1 knockout in neurons resulted in pathological vascular proliferation (20). These findings indicate that Sirt1 also regulates the normal morphogenesis of retinal blood vessels.
It has been confirmed that FOXO3 plays important regulatory roles in the proliferation, differentiation and cell cycle progression of some cell types. The role of FOXO3 has been examined in various vascular diseases and tumors (21,22). Sirt1 can regulate FOXO3 expression and transcription (23). The Sirt1/FOXO3 pathway plays a key role in the cellular adaptation and survival under hypoxic conditions and represents one of the molecular mechanisms underlying the energy restriction-mediated anti-aging effects. This pathway may serve as a new therapeutic target in the treatment of age- and hypoxia-related injuries (24). Sirt1 is responsible for the regulation of cell stress responses and cell survival by regulating FOXO3 (25-27). There is evidence showing Sirt1 may exert a dual regulatory effect on FOXO3 in case of oxidative stress. On one hand, Sirt1 enhances the ability of FOXO3 to induce cell cycle arrest and counteract with the reactive oxygen species (ROS) induced oxidative stress. On the other hand, Sirt1 reduces the ability of FOXO3 to induce apoptosis. Therefore, the Sirt1/FOXO3 pathway plays an important role in cell survival in oxidative stress (28).
Oxidative stress and endothelial dysfunction are involved in the pathogenesis of DR and other diabetic microangiopathies such as diabetic neuropathy and nephropathy. Yerra et al. found Sirt1 inactivation underlined the pathogenesis of high glucose-induced mitochondrial dysfunction and autophagic impairment in the experimental models of diabetic neuropathy. Sirt1 activation, PGC-1α-mediated mitochondrial biogenesis, and FOXO3a mediated stress resistance affected to counteract the multiple manifestations in experimental diabetic neuropathy (29). Resveratrol could modulate the SIRT1/FOXO3a pathway by increasing SIRT1 deacetylase activity and subsequently ameliorating renal tubular damage secondary to hyperglycemia-induced oxidative stress, which may be helpful for the development of new therapies for oxidative stress- related diabetic nephropathy (30).
In the present study, HUVECs were used. Destruction of blood-retinal barrier function due to the functional decompensation of retinal vascular endothelial cells represents one of the clinical manifestations of DR and the main pathological cause of blindness. HUVECs are commonly used in the studies on vascular endothelial cells. Compared with animal vascular endothelial cells, HUVECs are more representative in human body. HUVECs have been used in studies on endothelial cells in vitro (31). The present study focused on the roles of Sirt1/FOXO3 pathway in various biological functions of vascular endothelial cells under high-glucose conditions. Our results showed, under high-glucose conditions, the expression of Sirt1 and FOXO3 decreased without significant difference in HUVECs, the proliferative and migratory capabilities of HUVECs reduced, and apoptotic HUVEC increased. Given these findings, Sirt1 silencing, Sirt1 over-expression and Sirt1 agonist were employed in this study. Our results showed Sirt1 over-expression or Sirt1 agonist under high-glucose conditions reduced the high-glucose-mediated inhibition of proliferation and migration of HUVECs and decreased their apoptosis. In contrast, silencing of Sirt1 expression further enhanced high-glucose-mediated inhibition of proliferation and migration of HUVECs and increased their apoptosis. Some in vitro studies have shown the Sirt1 activity in rat heart, liver, lung, kidney and muscle is related to aging, and it decreases significantly as age increases (32,33). Many experiments have confirmed that Sirt1 prevents replicative senescence in mammalian cells (34). In contrast, selective knockout of Sirt1 at an early stage may slow cell growth and accelerate cell senescence (35). Our results showed Sirt1 expression and activity were regulated by Sirt1 over-expression or agonist under high-glucose conditions, and FOXO3 expression changed accordingly with Sirt1 expression. FOXO3 expression increased as Sirt1 expression increased, which was accompanied by the enhanced cellular function. These effects were reversed after silencing of FOXO3 expression. These findings demonstrate that Sirt1 exerts a regulatory effect on FOXO3 under high-glucose conditions. Both Sirt1 and FOXO3 are involved in diabetic vascular endothelial cell injuries.
PGC-1α is a transcriptional factor that modulates energy metabolism. PGC-1α plays important roles in regulating mitochondrial function, hepatic gluconeogenesis, glucose transport, insulin sensitivity and adaptive thermogenesis in the skeletal muscles. In addition, PGC-1α can regulate VEGF-A promoter activity, the expression of PGC-1α increases in the retina of DM animals, and inhibition of PGC-1α expression is able to down-regulate VEGF expression, thereby exerting protective effects on the retina (36). PGC-1α is a key downstream factor of Sirt1/FOXO3 pathway (9,37). In endothelial cells, FOXO3 may regulate the expression of some anti-oxidative genes via interacting with PGC-1α (38). Our results showed PGC-1α expression significantly increased in high-glucose (30 mM glucose) group compared with normal control group (5 mM glucose). Compared with normal control group, Sirt1 expression remained at a high level after inhibition of Sirt1 expression under high-glucose conditions. However, inhibition of Sirt1 expression under high-glucose conditions down-regulated PGC-1α expression compared with high glucose (30 mM glucose) group. In the high glucose environment, Sirt1 over-expression alone, Sirt1 over-expression plus FOXO3 inhibition, and Srit1 agonist plus PGC-1α inhibition resulted in the down-regulation of PGC-1α expression in HUVECs. Detection of FOXO3 activity further confirmed Sirt1/FOXO3 pathway was able to regulate PGC-1α expression under high-glucose conditions. Therefore, we speculate Sirt1/FOXO3 pathway is involved in the regulation of vascular endothelial cell function under high-glucose conditions by regulating PGC-1α. However, the specific underlying mechanisms are needed to be further investigated.
However, these were limitations in this study. HUVECs are derived from umbilical vein, which is a large blood vessel. Therefore, they can’t completely represent the intraocular capillaries. Further experiments on retinal vascular endothelial cells are needed in the future. The present study provided evidence on the Sirt1/FOXO3 pathway as a therapeutic target in the prevention and treatment of vascular endothelial cell injuries in high-glucose environments. Future efforts should focus on whether these effects are also present in the development of diabetes-induced retinal vascular endothelial injury in vivo.
Taken together, the Sirt1/FOXO3 pathway is essential for the survival of endothelial cells under high-glucose conditions and plays an important role in the development of diabetes-induced retinal vascular endothelial injury.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation, China (81300771, 81400397), the Natural Science Foundation of Shanghai (17ZR1427100), the Project of Key Disciplines of Medicine in Yangpu District of Shanghai (YP16ZA02), and the Scientific Research Foundation of Shanghai Municipal Commission of Health and Family Planning (201840336).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bunce C, Wormald R. Leading causes of certification for blindness and partial sight in England & Wales. BMC Public Health 2006;6:58. [Crossref] [PubMed]
- Shin ES, Sorenson CM, Sheibani N. Diabetes and retinal vascular dysfunction. J Ophthalmic Vis Res 2014;9:362-73. [PubMed]
- Xiong S, Salazar G, Patrushev N, et al. Peroxisome proliferator-activated receptor gamma coactivator-1alpha is a central negative regulator of vascular senescence. Arterioscler Thromb Vasc Biol 2013;33:988-98. [Crossref] [PubMed]
- Hao CM, Haase VH. Sirtuins and their relevance to the kidney. J Am Soc Nephrol 2010;21:1620-7. [Crossref] [PubMed]
- Cheng BB, Yan ZQ, Yao QP, et al. Association of SIRT1 expression with shear stress induced endothelial progenitor cell differentiation. J Cell Biochem 2012;113:3663-71. [Crossref] [PubMed]
- Potente M, Ghaeni L, Baldessari D, et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev 2007;21:2644-58. [Crossref] [PubMed]
- Li F, Xie P, Fan Y, et al. C terminus of Hsc70-interacting protein promotes smooth muscle cell proliferation and survival through ubiquitin-mediated degradation of FoxO1. J Biol Chem 2009;284:20090-8. [Crossref] [PubMed]
- Thors B, Halldorsson H, Thorgeirsson G. eNOS activation mediated by AMPK after stimulation of endothelial cells with histamine or thrombin is dependent on LKB1. Biochim Biophys Acta 2011;1813:322-31. [Crossref] [PubMed]
- Olmos Y, Sanchez-Gomez FJ, Wild B, et al. SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1alpha complex. Antioxid Redox Signal 2013;19:1507-21. [Crossref] [PubMed]
- Cantó C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 2009;20:98-105. [Crossref] [PubMed]
- Zhou S, Chen HZ, Wan YZ, et al. Repression of P66Shc expression by SIRT1 contributes to the prevention of hyperglycemia-induced endothelial dysfunction. Circ Res 2011;109:639-48. [Crossref] [PubMed]
- Milne JC, Lambert PD, Schenk S, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007;450:712-6. [Crossref] [PubMed]
- Zeng L, Chen R, Liang F, et al. Silent information regulator, Sirtuin 1, and age-related diseases. Geriatr Gerontol Int 2009;9:7-15. [Crossref] [PubMed]
- Fry JL, Shiraishi Y, Turcotte R, et al. Vascular Smooth Muscle Sirtuin-1 Protects Against Aortic Dissection During Angiotensin II-Induced Hypertension. J Am Heart Assoc 2015;4:e002384. [Crossref] [PubMed]
- Stein S, Matter CM. Protective roles of SIRT1 in atherosclerosis. Cell Cycle 2011;10:640-7. [Crossref] [PubMed]
- Csiszar A, Labinskyy N, Podlutsky A, et al. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol 2008;294:H2721-35. [Crossref] [PubMed]
- Kowluru RA, Santos JM, Zhong Q. Sirt1, a negative regulator of matrix metalloproteinase-9 in diabetic retinopathy. Invest Ophthalmol Vis Sci 2014;55:5653-60. [Crossref] [PubMed]
- Mortuza R, Feng B, Chakrabarti S. miR-195 regulates SIRT1-mediated changes in diabetic retinopathy. Diabetologia 2014;57:1037-46. [Crossref] [PubMed]
- Kubota S, Ozawa Y, Kurihara T, et al. Roles of AMP-activated protein kinase in diabetes-induced retinal inflammation. Invest Ophthalmol Vis Sci 2011;52:9142-8. [Crossref] [PubMed]
- Chen J, Michan S, Juan AM, et al. Neuronal sirtuin1 mediates retinal vascular regeneration in oxygen-induced ischemic retinopathy. Angiogenesis 2013;16:985-92. [Crossref] [PubMed]
- Li Y, Yang G, Yang X, et al. Nicotinic acid inhibits vascular inflammation via the SIRT1-dependent signaling pathway. J Nutr Biochem 2015;26:1338-47. [Crossref] [PubMed]
- Lin CH, Chang CY, Lee KR, et al. Flavones inhibit breast cancer proliferation through the Akt/FOXO3a signaling pathway. BMC Cancer 2015;15:958. [Crossref] [PubMed]
- Ramadori G, Gautron L, Fujikawa T, et al. Central administration of resveratrol improves diet-induced diabetes. Endocrinology 2009;150:5326-33. [Crossref] [PubMed]
- Kume S, Uzu T, Horiike K, et al. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest 2010;120:1043-55. [Crossref] [PubMed]
- Motta MC, Divecha N, Lemieux M, et al. Mammalian SIRT1 represses forkhead transcription factors. Cell 2004;116:551-63. [Crossref] [PubMed]
- Wang F, Nguyen M, Qin FX, et al. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell 2007;6:505-14. [Crossref] [PubMed]
- Wang YQ, Cao Q, Wang F, et al. SIRT1 Protects Against Oxidative Stress-Induced Endothelial Progenitor Cells Apoptosis by Inhibiting FOXO3a via FOXO3a Ubiquitination and Degradation. J Cell Physiol 2015;230:2098-107. [Crossref] [PubMed]
- Brunet A, Sweeney LB, Sturgill JF, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004;303:2011-5. [Crossref] [PubMed]
- Yerra VG, Kalvala AK, Kumar A. Isoliquiritigenin reduces oxidative damage and alleviates mitochondrial impairment by SIRT1 activation in experimental diabetic neuropathy. J Nutr Biochem 2017;47:41-52. [Crossref] [PubMed]
- Wang X, Meng L, Zhao L, et al. Resveratrol ameliorates hyperglycemia-induced renal tubular oxidative stress damage via modulating the SIRT1/FOXO3a pathway. Diabetes Res Clin Pract 2017;126:172-81. [Crossref] [PubMed]
- Dal Monte M, Rezzola S, Cammalleri M, et al. Antiangiogenic Effectiveness of the Urokinase Receptor-Derived Peptide UPARANT in a Model of Oxygen-Induced Retinopathy. Invest Ophthalmol Vis Sci 2015;56:2392-407. [Crossref] [PubMed]
- Braidy N, Guillemin GJ, Mansour H, et al. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS One 2011;6:e19194. [Crossref] [PubMed]
- Lee D, Goldberg AL. SIRT1 protein, by blocking the activities of transcription factors FoxO1 and FoxO3, inhibits muscle atrophy and promotes muscle growth. J Biol Chem 2013;288:30515-26. [Crossref] [PubMed]
- Yamashita S, Ogawa K, Ikei T, et al. SIRT1 prevents replicative senescence of normal human umbilical cord fibroblast through potentiating the transcription of human telomerase reverse transcriptase gene. Biochem Biophys Res Commun 2012;417:630-4. [Crossref] [PubMed]
- Helmrich U, Marsano A, Melly L, et al. Generation of human adult mesenchymal stromal/stem cells expressing defined xenogenic vascular endothelial growth factor levels by optimized transduction and flow cytometry purification. Tissue Eng Part C Methods 2012;18:283-92. [Crossref] [PubMed]
- Zheng Z, Chen H, Wang H, et al. Improvement of retinal vascular injury in diabetic rats by statins is associated with the inhibition of mitochondrial reactive oxygen species pathway mediated by peroxisome proliferator-activated receptor gamma coactivator 1alpha. Diabetes 2010;59:2315-25. [Crossref] [PubMed]
- Zarzuelo MJ, Lopez-Sepulveda R, Sanchez M, et al. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochem Pharmacol 2013;85:1288-96. [Crossref] [PubMed]
- Olmos Y, Valle I, Borniquel S, et al. Mutual dependence of Foxo3a and PGC-1alpha in the induction of oxidative stress genes. J Biol Chem 2009;284:14476-84. [Crossref] [PubMed]


