
Malnutrition in patients who underwent surgery for spinal metastases
Introduction
The spine is the most common site for bony metastases, with up to 70% of the patients with advanced cancer developing spinal metastases (1). Metastatic spine disease can result in debilitating pain and increases the risk for pathological fracture, neurological deficits, spinal cord compression and spinal instability. Proper management of metastatic spinal disease is therefore essential. Radiotherapy has been the standard of care for the treatment of uncomplicated painful spinal metastases with surgery generally reserved for patients with pain caused by mechanical instability and/or symptomatic spinal cord compression (2). Although surgery has shown to result in significant improvements in health-related quality of life (HRQOL), it has also been associated with potential high rates of adverse events (3-6). Due to the palliative nature of the procedures, the benefits of surgery should outweigh the risks; accurate prediction of survival and associated surgical risks are therefore important as it may assist in determining the type and extent of treatment offered. In order to optimize treatment selection, we need to identify prognostic factors that are ideally easy to assess, reliable and modifiable.
Nutritional status as a prognostic factor has been gaining attention in the oncology literature (7-10). Malnutrition has been reported in 40% to 80% of the cancer patients, and has been associated with profound consequences such as increased morbidity, mortality, risk of complications, length of stay and decreased patient reported treatment outcomes (11-14). Malnutrition in a cancer patient is complex and is influenced by different factors including physical symptoms, nutritional intake, caloric need, systemic inflammation, muscle mass depletion and treatment-related side effects (9), which are not simply managed by increasing caloric intake. Historically, nutritional status has been assessed using laboratory values, anthropometric measurements and various scoring systems, reflecting the lack of consensus for a gold standard for assessing nutritional status and defining malnutrition. More recently, the Patient-Generated Subjective Global Assessment (PG-SGA) is increasingly being used to assess patients’ nutritional status and has been validated in the oncology population (13,15).
To date there is a paucity of literature regarding the nutritional status of patients with spinal metastases and its potential prognostic significance. The primary objective of this study was, therefore, to evaluate the nutritional status of patients who underwent surgical treatment for spinal metastases. In addition, the association between the pre-operative nutritional status and length of stay, HRQOL, the occurrence of adverse events and survival was explored.
Methods
A single center (University Medical Center Utrecht, The Netherlands) prospective observational cohort study of patients with spinal metastases who were over the age of 18 and underwent surgical stabilization with or without postoperative radiotherapy for spinal metastases was performed. Patients were not eligible for inclusion if they were diagnosed with a primary spinal bone tumor or a primary central nervous system tumor, or were unable to complete the nutritional assessment. The local institutional review board approved the research protocol and all patients provided written informed consent.
Data regarding demographics, primary tumor diagnosis, surgical and/or radiotherapy treatment, neurological status [American Spinal Injury Association (ASIA) score] (16), Karnofsky performance (17) status, adverse events and survival were prospectively collected. Governmental databases were accessed to retrieve information regarding survival. Quality of life was evaluated at baseline, 6- and 12-week follow-up using the Spine Oncology Study Group Outcomes Questionnaire (SOSGOQ2.0) and the Euroqol five dimensions (EQ-5D-3L). The SOSGOQ2.0 is a spine oncologic specific HRQOL measure, which includes 20 items divided in five domains including physical functioning, mental health, pain, neurological function and social functioning (18,19). A higher score on the SOSGOQ and the EQ-5D-3L represents a better quality of life. The EQ-5D is a generic HRQOL measure based on the evaluation of five HRQOL dimensions.
Nutritional status was evaluated at baseline using the short-form of the PG-SGA (13). The short form of the PG-SGA is completed by the patient and includes an assessment of weight, weight change, nutritional intake, physical symptoms and performance status. The sum of the different assessments results in a numeric risk score, with a higher score denoting a greater risk for malnutrition and a need for dietary consultation. A score of 9 or above denotes a critical need for a nutritional intervention. In addition to the numeric score, patients were assigned a PG-SGA global score based on the short form assessments (13,15). Three PG-SGA global scores can be distinguished indicating: (I) a well-nourished patient, (II) suspected malnutrition and (III) malnutrition.
Statistics
Demographic and HRQOL data were summarized using descriptive statistics (mean and standard deviation (SD) or median and range for continuous variables; absolute number and frequency for categorical variables). The independent samples t-test, Wilcoxon rank sum test and one-way ANOVA were used to compare differences in continuous data; Chi-square and Fisher’s exact tests were used for categorical data. The SOSGOQ2.0 and EQ-5D-3L scores were analyzed by their total scores and by their subdomains respectively. Univariate logistic regression and linear regression were used to determine the association between nutritional status and the occurrence of complications, length of stay, EQ-5D scores and SOSGOQ2.0 scores and survival. Kaplan-Meier survival curves were drawn and patients who did not reach the 12 weeks follow-up time point were censored. The log-rank test was used to compare survival between the three PG-SGA categories. Cox proportional hazards analyses were used to assess the correlation between the numerical PGSGA risk scores and survival. Given the descriptive and explorative nature of the study no ante hoc sample size calculation was performed. All statistical analyses were performed using IBM SPSS statistics for Macintosh, version 24.0 (Armonk, NY: IBM Corp). The significance level was set at P<0.05.
Results
Demographics
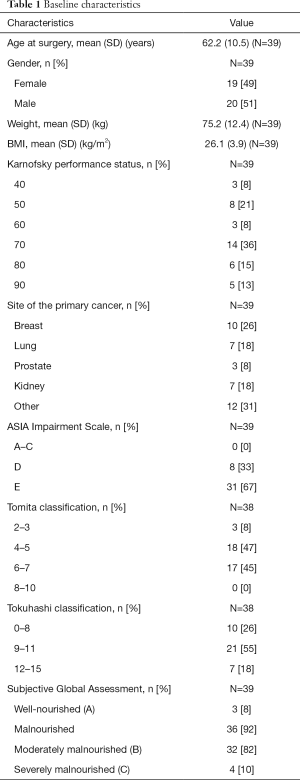
A total of 39 patients were included in the final analysis; 19 patients were male (49%) and the mean age was 62.2 years (SD 10.5). The most common primary tumor was breast cancer (N=10) followed by renal cell cancer (N=7) and lung cancer (N=7). Pre-operatively, 31 patients presented neurologically intact (ASIA E), eight patients presented with ASIA D and none of patients presented with ASIA A-C. Baseline characteristics of the study population are displayed in Table 1.
Full table
Surgical details
A posterior surgical approach was used in all operations. Five or more vertebral bodies were instrumented in 14 patients (36%), four vertebral bodies in three patients (8%), and three vertebral bodies in 21 patients (54%). One patient underwent cement augmentation of a single vertebral body (3%). The median operating time was 120 minutes (range, 60–180 minutes); median blood loss was 100 mL (range, 50–1,400 mL). Fourteen patients received post-operative adjuvant radiotherapy; the most common fractionation schedule was 10×3 Gy in nine patients, followed by 1×8 Gy in three patients, and 5×4 Gy in one patient.
Nutritional status
According to the PG-SGA short form assessment, 11 of 39 patients did not require a dietary intervention (score 0–3), 28 required a dietary intervention of which in 10 patients a critical need for an intervention was indicated (score of 9 or higher). Based on the PG-SGA global scores, only 3 of 39 patients were well-nourished (global score A) and 36 (92%) were malnourished. Of the malnourished patients, 32 (82%) patients were moderately or suspected of being malnourished (global score B) and 4 (10%) patients were severely malnourished (global score C). Nutritional intake problems (e.g., less than usual or liquid food) were reported by 74% of the patients; 50% of the patients who had no nutritional intake problems reported the presence of two or more physical symptoms, such as vomiting or mouth sores, impairing nutritional intake.
The median body mass index (BMI) for patients with a PG-SGA global score A was 24.4 (range, 22.9–32.6), compared to a median BMI of 25.2 (range, 19.2–35.9) for patients with a PG-SGA global score B and a median BMI of 22.8 (range, 22.3–29.7) for patients with a PG-SGA global score C.
HRQOL
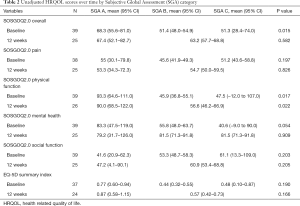
At baseline, the mean SOSGOQ2.0 overall score was 68.3, 51.4, and 51.3 for patients with a PG-SGA global score A, B, and C (P=0.015), respectively. In addition, malnourished patients had worse baseline SOSGOQ2.0 physical function, social function and mental health scores compared to well-nourished patients. Comparison across all PG-SGA global scores at 12 weeks post-surgery, showed a mean SOSGOQ2.0 overall score of 67.4 (N=3) for PG-SGA A patients, and a score of 63.2 (N=22) for the PG-SGA B and C patients combined (P=0.582) (Table 2).
Full table
Based on the numeric nutritional risk score, the mean SOSGOQ2.0 score was 57.7, 50.0, and 52.4 for patients with a PG-SGA score between 0–3, 4–8, and 9 or above (P=0.133), respectively. Comparison across all PG-SGA scores 12 weeks post-surgery, showed a mean overall SOSGOQ2.0 score of 63.9 (N=7) for patients with a PG-SGA score between 0–4, a score of 63.4 (N=12) for patients with a PG-SGA score between 5–8 and 64.2 (N=5) for patients with a PG-SGA score of 9 or above (P=0.990).
At baseline, the mean EQ-5D-3L summary index was 0.77, 0.44, and 0.48 for PG-SGA A, B, and C (P=0.190), respectively. In addition, the impairment in the ability to perform daily activities was greater in malnourished compared to well-nourished patients (P=0.080). Comparison across the PG-SGA global scores 12 weeks post-surgery, showed a mean EQ-5D-3L summary index of 0.87 (N=3) for PG-SGA A patients and a mean index of 0.57 (N=21) for PG-SGA B and C patients combined (P=0.166) (Table 2).
At baseline, the mean EQ-5D-3L summary index was 0.56, 0.49, and 0.32 for patients with a PG-SGA score between 0–3, 4–8, and 9 or above (P=0.226), respectively. Comparison across all PG-SGA scores at 12 weeks post-surgery, showed a mean EQ-5D-3L score of 0.68 (N=7) for patients with PG-SGA score between 0–3, a mean index of 0.65 (N=12) for patients with a PG-SGA score between 4–8 and 0.46 (N=5) for patients with a PG-SGA score of 9 or above (P=0.495).
Treatment outcomes
A total of six adverse events were documented in six patients. Adverse events included neurological deterioration in two patients, a pulmonary embolism, transient urinary retention, a pathological femur fracture and symptomatic humeral metastases. Overall median length of hospital stay was 6 days (range, 1–100 days). No association between nutritional status and the occurrence of adverse events or length of hospital stay could be determined. The median follow-up for survival was 18 months; at last follow-up only 23 patients (59%) were still alive. Patients presenting with a PG-SGA score between 0 and 8 had a mean survival of 33.4 months compared to 21.5 months for those presenting with a PG-SGA score of 9 or above (P=0.386). The mean survival time was 32.8 months for PG-SGA B patients and 26.6 months for PG-SGA C patients (P=0.451). Both PG-SGA A patients were alive at final follow-up with a mean follow-up of 20.3 months.
Discussion
As spinal metastases normally indicate advanced disease, treatment goals for patients with spinal metastases shift from cure to comfort, with the primary goals of relieving pain and maintaining or improving HRQOL. Symptom management is herein essential, as symptom control has been associated with improved HRQOL but also with improved survival (20,21). Pain, treatment related side effects and other physical symptoms may all impair nutritional intake, leading to malnutrition. Malnutrition is common among cancer patients and has been associated with increased morbidity and mortality, and significantly contributes to the disease burden (11-14). Nutritional status is potentially modifiable by adequate nutritional support and could thereby improve HRQOL and treatment outcomes. The purpose of this study was to obtain preliminary data regarding the nutritional status of patients with spinal metastases who underwent surgical treatment. In addition, we aimed to explore the potential relationship between nutritional status and length of stay, adverse events, HRQOL and survival. We demonstrated that according to the PG-SGA, 92% (N=36) of our patients were moderately or severely malnourished. Based on the numeric nutritional risk score, 72% of the patients required a nutritional intervention. Malnourishment was associated with lower HRQOL scores at baseline. However, no statistically significant association between malnutrition and the occurrence of adverse events, length of stay, survival or post-treatment HRQOL could be determined in the present study.
Several studies have reported worse HRQOL scores in patients with malnutrition as compared to well-nourished patients. In a retrospective study Gupta et al. evaluated the association between nutritional status, including the SGA, and HRQOL with the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (QLQ-C30) in 58 patients with stage III–IV colorectal cancer (22). The authors demonstrated that well-nourished patients had significantly better HRQOL scores on the global, physical, pain and role scales of the QLQ-C30 as compared to malnourished patients (22). These results are in agreement with the results from our study, which showed lower baseline SOSGOQ2.0 total and SOSGOQ2.0 physical function scores in malnourished patients as compared to well-nourished patients. Although statistically non-significant, differences between well-nourished and malnourished patients in EQ-5D total scores, EQ-5D usual activity levels, SOSGOQ2.0 mental health and social function were clinically relevant. No difference in pain could be determined between malnourished and well-nourished patients in our study.
Our results found no association between malnutrition as defined by the PG-SGA global score or the PG-SGA nutritional risk score and survival. This is in contrast with other previously published studies on the association between malnutrition and survival in other oncology populations. Tan et al. conducted a prospective cohort study in 114 patients with advanced cancer and demonstrated a significant association between malnutrition and mortality (7). Severely malnourished patients (PG-SGA C) had a median survival of 5.6 months, as compared to a median survival of 11.1 months for moderately malnourished patients (PG-SGA B) and a median survival of 18.8 months for well-nourished patients (PG-SGA A) (7). In comparison, the mean survival time of severely malnourished patients in our study was 26.6 months as compared to 32.8 months for moderately malnourished patients, which showed no statistically significant difference. This difference in statistical significance may be explained by our limited sample size resulting in a potential type 2 statistical error, and the skewed distribution of the patients among the different PG-SGA categories, with the majority of the patients being classified as moderately malnourished.
According to the PG-SGA global score, 82% of the patients in our study were moderately malnourished and 10% were severely malnourished. Although it is known that malnutrition is common among cancer patients, malnutrition rates among patients with spinal metastases were previously unknown. In comparison, in the study of Gupta et al. that included stage III–IV colorectal cancer patients, 59% of the patients were well-nourished, 38% were moderately malnourished and 3% were severely malnourished. The nutritional status of the patients in the present study was evaluated with the short form of the PG-SGA. Historically, many different questionnaires, laboratory values and anthropometric measurements have been used to evaluate the nutritional status (23,24). The PG-SGA was specifically designed for and validated within the oncology population and has been accepted by the Oncology Nutrition Dietetic Practice Group of the American Dietetic Association as the gold standard measure for nutritional risk assessment in cancer patients (15). The full PG-SGA consists of two parts, one part that is completed by the patient and the second part that is completed by a physician or trained dietician. The part that is completed by the patient is also referred to as the PG-SGA short form. The PG-SGA short form has also been shown to be a valid measure for the evaluation of nutritional status and has been associated with length of stay, HRQOL, and survival (25). An advantage of using the (short-form) PG-SGA rather than anthropometric measurements or laboratory values is that the PG-SGA is able to identify symptoms that impair adequate nutritional intake thereby enabling direct dietary or other medical interventions. In addition, the PG-SGA is likely more sensitive to changes over a short period of time as compared to laboratory values.
A major limitation of this study was the limited sample size. However, enrolment and follow-up of patients with spinal metastases in cohort studies is challenging given their limited life expectancy. An explorative statistical approach was used rather than an ante hoc sample size calculation as a result the sample size limited the ability to perform extensive analyses and is likely to be underpowered to detect smaller, but still clinically relevant, statistically significant differences in HRQOL, survival and the occurrence of adverse events between the different categories of malnutrition. In addition, this study was conducted in a single tertiary care center limiting the generalizability of the results. Finally, the effects of a nutritional intervention to improve nutritional status or symptoms were not evaluated in our study. The PG-SGA was used solely for observational research purposes; patients might have received nutritional interventions based on the discretion of their treating physician (e.g., medical oncologist), which might have influenced the results.
Inadequate nutritional intake prohibits maintenance of muscle mass, thereby significantly contributing to the development of sarcopenia and frailty. Sarcopenia is defined as the loss of muscle mass, combined with a decline in strength and/or muscle function (26). Frailty is a complex syndrome, reflecting a state of increased vulnerability to effects of stressors and increased risk of adverse health outcomes (27). Frailty may be appreciated as the accumulation of functional deficits, which may be expressed by an index. The Metastatic Spinal Tumour Frailty Index (MSTFI) was developed in response to the lack of data regarding frailty and clinical outcomes in patients with spinal metastases (28). This index was developed using a large nationwide database and includes nine independent parameters that were significantly associated with adverse events and survival (28). One of the parameters included in the MSTFI is malnutrition. The odds for the occurrence of an adverse event among malnourished patients was 2.11 times the odds of the occurrence of an adverse event of well-nourished patients, emphasizing the importance of assessing malnutrition in this patient population (28).
Conclusions
This study is the first to demonstrate that malnutrition as determined by the PG-SGA short is highly prevalent among patients with spinal metastases. In addition, malnutrition was associated with lower baseline SOSGOQ2.0 overall scores, SOSGOQ2.0 physical functioning scores, mental health, and social functioning scores, as well as lower baseline overall EQ-5D scores and EQ-5D usual activity scores. No significant association between malnutrition and the occurrence of adverse events, length of stay, post-treatment HRQOL or survival could be determined. The results of this study support the need for future larger studies to evaluate the prognostic significance of malnutrition and the influence of nutritional interventions in patients with spinal metastases.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The local institutional review board approved the research protocol (No. 15-475) and all patients provided written informed consent.
References
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006;12:6243s-6249s. [Crossref] [PubMed]
- Barzilai O, Laufer I, Yamada Y, et al. Integrating Evidence-Based Medicine for Treatment of Spinal Metastases into a Decision Framework: Neurologic, Oncologic, Mechanicals Stability, and Systemic Disease. J Clin Oncol 2017;35:2419-27. [Crossref] [PubMed]
- Dea N, Versteeg A, Fisher C, et al. Adverse events in emergency oncological spine surgery: a prospective analysis. J Neurosurg Spine 2014;21:698-703. [Crossref] [PubMed]
- Falicov A, Fisher CG, Sparkes J, et al. Impact of surgical intervention on quality of life in patients with spinal metastases. Spine 2006;31:2849-56. [Crossref] [PubMed]
- Versteeg AL, Verlaan JJ, de Baat P, et al. Complications After Percutaneous Pedicle Screw Fixation for the Treatment of Unstable Spinal Metastases. Ann Surg Oncol 2016;23:2343-9. [Crossref] [PubMed]
- Fehlings MG, Nater A, Tetreault L, et al. Survival and Clinical Outcomes in Surgically Treated Patients With Metastatic Epidural Spinal Cord Compression: Results of the Prospective Multicenter AOSpine Study. J Clin Oncol 2016;34:268-76. [Crossref] [PubMed]
- Tan CS, Read JA, Phan VH, et al. The relationship between nutritional status, inflammatory markers and survival in patients with advanced cancer: a prospective cohort study. Support Care Cancer 2015;23:385-91. [Crossref] [PubMed]
- Mantzorou M, Koutelidakis A, Theocharis S, et al. Clinical Value of Nutritional Status in Cancer: What is its Impact and how it Affects Disease Progression and Prognosis? Nutr Cancer 2017;69:1151-76. [Crossref] [PubMed]
- Read JA, Choy ST, Beale PJ, et al. Evaluation of nutritional and inflammatory status of advanced colorectal cancer patients and its correlation with survival. Nutr Cancer 2006;55:78-85. [Crossref] [PubMed]
- Gupta D, Lis CG, Vashi PG, et al. Impact of improved nutritional status on survival in ovarian cancer. Support Care Cancer 2010;18:373-81. [Crossref] [PubMed]
- Ollenschläger G, Viell B, Thomas W, et al. Tumor anorexia: causes, assessment, treatment. Recent Results Cancer Res 1991;121:249-59. [Crossref] [PubMed]
- Kern KA, Norton JA. Cancer cachexia. JPEN J Parenter Enteral Nutr 1988;12:286-98. [Crossref] [PubMed]
- Ottery FD. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition 1996;12:S15-9. [Crossref] [PubMed]
- Gupta D, Vashi PG, Lammersfeld CA, et al. Role of Nutritional Status in Predicting the Length of Stay in Cancer: A Systematic Review of the Epidemiological Literature. Ann Nutr Metab 2011;59:96-106. [Crossref] [PubMed]
- Bauer J, Capra S, Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr 2002;56:779-85. [Crossref] [PubMed]
- Kirshblum SC, Burns SP, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med 2011;34:535-46. [Crossref] [PubMed]
- Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: Macleod CM. editor. Evaluation of chemotherapeutic agents. New York: Columbia University Press, 1949:199-205.
- Street J, Lenehan B, Berven S, et al. Introducing a new health-related quality of life outcome tool for metastatic disease of the spine: content validation using the International Classification of Functioning, Disability, and Health; on behalf of the Spine Oncology Study Group. Spine 2010;35:1377-86. [Crossref] [PubMed]
- Versteeg AL, Sahgal A, Rhines LD, et al. Psychometric evaluation and adaptation of the Spine Oncology Study Group Outcomes Questionnaire to evaluate health-related quality of life in patients with spinal metastases. Cancer 2018;124:1828-38. [Crossref] [PubMed]
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-42. [Crossref] [PubMed]
- Vigano AL, di Tomasso J, Kilgour RD, et al. The abridged patient-generated subjective global assessment is a useful tool for early detection and characterization of cancer cachexia. J Acad Nutr Diet 2014;114:1088-98. [Crossref] [PubMed]
- Gupta D, Lis CG, Granick J, et al. Malnutrition was associated with poor quality of life in colorectal cancer: a retrospective analysis. J Clin Epidemiol 2006;59:704-9. [Crossref] [PubMed]
- Kyle UG, Kossovsky MP, Karsegard VL, et al. Comparison of tools for nutritional assessment and screening at hospital admission: a population study. Clin Nutr 2006;25:409-17. [Crossref] [PubMed]
- Isenring EA, Banks M, Ferguson M, et al. Beyond malnutrition screening: appropriate methods to guide nutrition care for aged care residents. J Acad Nutr Diet 2012;112:376-81. [Crossref] [PubMed]
- Sealy MJ, Haß U, Ottery FD, et al. Translation and Cultural Adaptation of the Scored Patient-Generated Subjective Global Assessment: An Interdisciplinary Nutritional Instrument Appropriate for Dutch Cancer Patients. Cancer Nurs 2018;41:450-62. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412-23. [Crossref] [PubMed]
- Wei K, Nyunt MS, Gao Q, et al. Association of Frailty and Malnutrition With Long-term Functional and Mortality Outcomes Among Community-Dwelling Older Adults: Results From the Singapore Longitudinal Aging Study 1. JAMA Netw Open 2018;1:e180650. [Crossref] [PubMed]
- De la Garza Ramos R, Goodwin CR, Jain A, et al. Development of a Metastatic Spinal Tumor Frailty Index (MSTFI) Using a Nationwide Database and Its Association with Inpatient Morbidity, Mortality, and Length of Stay After Spine Surgery. World Neurosurg 2016;95:548-555.e4. [Crossref] [PubMed]


