
Advances in personalized treatment of metastatic spine disease
Introduction
The spine is one of the most common sites of bony metastases (1), where cadaveric studies have shown that 30–90% of patients diagnosed with cancer will have spinal metastases by time of death (2). Metastatic spine tumors commonly originate from solid tumors of the lung (3), prostate (4), breast (5) and non-solid hematological sources. Due to its vital role in providing postural support, movement, and protection of the spinal cord and nerve roots, metastatic spine disease often leads to significant morbidity in patients. Disruption of normal bone turnover by local osteogenic or osteolytic effects of metastatic tumors creates a detrimental instability in the weight bearing spinal column. This in turn can lead to pain, pathological fractures, and ultimately neurological deficits through involvement of adjacent neurovascular structures (6). Advancements in management of patients with metastatic disease has led to increased survivability over the past decades, making effective management of spinal metastases critical to improving their quality of life throughout the course of their illness (7-9).
Current modalities for management of metastatic spine disease include radiotherapy, surgery and systemic chemo/antiresorptive therapy (10). However, surgery has proved to be the most effective intervention in patients with neurological deficits and bony instability (11,12). In 2005, Patchell et al. (11) published a landmark article that solidified the role of surgery in the management of spinal metastasis. In this study, patients with metastatic epidural spinal cord compression were randomized to undergo surgical decompression in addition to radiation therapy or radiation therapy alone. The study was terminated early as surgery together with radiation therapy was shown to be superior to radiation therapy alone in the interim analysis. Regarding spine instability, the development of the Spinal Instability Neoplastic Score (SINS) in 2010 has helped define spinal instability in a reproducible manner and to guide surgical decision making (12-14).
The basis of surgical interventions in metastatic spine disease involves removing or debulking a tumor to alleviate its biochemical and mass effects on the surrounding bony and neurovascular tissue. This can be followed by instrumented fusion of adjacent segments to restore structural integrity to the spinal column (15). Removal of the tumor can lead to unstable critical size defects that limit natural bone remodelling, requiring use of bone substitutes to aid healing (16). This is not without risk, as more than 10% of these patients must be re-operated on often due to hardware failure or other complications (17,18), which can become a costly burden on the healthcare system (19). Additionally, tumor recurrence and continuous local bone loss require systemic chemo- and antiresorptive therapy which can cause significant systemic side-effects such as osteonecrosis of the jaw (20) or renal toxicity (21), limiting their prolonged use. Recent trends have demonstrated the potential of novel bone substitute materials for delivering therapeutics locally to avoid side-effects associated with systemic therapy while at the same time promoting bone regeneration. In summary, there is still a high reoperation rate with many being secondary to local recurrence (18). An understanding of the cellular and molecular basis of the metastatic process might help improve local control following spinal surgery.
Cellular and molecular basis of bone metastasis
Seed and soil hypothesis
While there are still significant questions about metastasis that have yet to be answered, the discoveries that will be discussed have led to changes in therapeutic approaches that have had a significant impact on patients’ outcomes. Currently, “the “seed and soil” hypothesis” on distant metastasis emphasises the importance of the interplay between both the tumor cells and target organ characteristics in the success of the metastatic process (22). This hypothesis, described by Dr. Paget in 1889 (22,23), posits that metastatic cells travel far from the primary site, bury themselves in the target organ, such as bone marrow, and lay dormant until certain conditions drive the cells to proliferate. This was based on observations that rates of metastases to different organs did not correlate with the relative share of the blood flow based on the autopsy reports of 735 patients with breast cancer (23). Paget also contrasted his findings in patients with breast cancer to those with other primary tumors. These observations were later confirmed by Hart et al. (24) in a metastatic melanoma rat model. Tissues from different organs (lung, kidney and ovary) were ectopically implanted in animal hind limbs, and a metastatic melanoma model was established by intravenous injection of tumor cells. The authors noted that melanoma tends to preferentially metastasize to pulmonary tissue and concluded that the process of metastases is not random. Studies have also shown that metastases can exert a selective pressure on tumor cells, thus resulting in metastatic tumors that differ from the original tumor (22,25-27). In 1970, Fidler showed that only 1.5% of melanoma tumor cells which enter the circulation will survive beyond 24 hours (25). Furthermore, Fidler et al. (27) showed that metastatic tumors are monoclonal in nature. In these experiments, 2 different melanoma cell lines were injected intravenously as either a homogenous or a heterogenous mixture. The resultant lung metastases were all found to have originated from a single cell line suggesting that only certain tumor cells can successfully metastasize (27). These findings could help explain why metastatic tumors might respond differently than the parent tumor when exposed to the same therapeutic agents.
Tumor cells that manage to survive and reach the skeleton must interact with a microenvironment unique from other organs. Furthermore, bone itself can be subclassified into cortical and cancellous, both of which differ in physical structure and metabolic activity. The bone, therefore, offers metastatic cells unique micro-environments that are termed “niches” (28). The impact of the physical characteristics of the micro-environment on tumor cell behavior was studied by Ruppender et al. (28,29). Using 2D models that simulated tissues with different structural rigidity, they were able to show that tumor cells expressed increasing levels of parathyroid hormone-related protein (PTHrP) with increasing rigidity of their physical environment (29). The rigidity in this test model was achieved through seeding cells on polyurethane (PUR) films that were under varying degrees of tensile stress (29). The gene responsible for PTHrP expression is GLI2, which is a zinc finger transcription factor which increases in expression with increased environmental stiffness (30-32). PTHrP/GLI2 may be a potential therapeutic target against skeletal-related complications of bone metastases. This was demonstrated by Gallwitz et al. (33) whereby inhibition of GLI2 transcription through guanine-nucleotide analog (6-Thioguanine) decreased PTHrP expression, PTHrP-induced osteolysis and hypercalcemia in a mouse metastasis model. These findings are significant given the fact that PTHrP is known to increase osteoclastic activity and bone resorption. Bone is also a dynamic structure that is being constantly remodeled in a process that couples bone resorption and bone formation (28). Bone resorption is carried out by osteoclasts while osteoblasts form new bone during the remodelling process (28). Bone remodelling centers tend to be rich in nutrients and growth factors compared to regions that are not undergoing remodeling. Tumor cells that metastasize to remodelling zones tend to have higher growth potential compared to those that metastasize to bone that is quiescent (28). Conditions and medications that increase bone turnover have been shown to increase metastatic tumor growth within the skeleton (28,34,35).
Stages of metastasis
The process of bone metastasis can be grouped into four stages: colonization, dormancy, reactivation and growth (28). Each of these stages varies based on the type of tumor cell and the type of niche where it arises (28). Colonization is the stage in which metastatic tumor cells enter the bone marrow (28). Studies have shown that colonization of the skeleton does not necessarily lead to overt metastatic boney lesions (28,36). Domschke studied the impact of finding disseminated tumor cells within a bone marrow biopsy in a cohort of 1,378 breast cancer patients (36). Of these, 621 patients had positive bone marrow biopsies, but only 139 (22.4%) ended up developing boney metastasis (36). The next step is dormancy in which tumor cells start to adapt to their new environment (28). For example, dormant multiple myeloma cells are more likely to be present in niches rich in osteoblastic cells (37). Another study showed that dormant multiple myeloma cells were resistant to melphalan, which is an alkylating agent used to treat multiple myeloma (38). These findings could help explain why patients can undergo early and late relapse following treatment. Tumor cells which are dormant can be reactivated by a variety of stimuli (28). While the exact mechanisms of reactivation are still being investigated, it is believed that osteoclasts play an important role by changing the biophysical environment within a niche and/or altering cell signalling pathways (28,38). Once released from dormancy, tumor cells form micro-metastases which then modify the local environment through cell signalling and ultimately result in overt metastasis (28).
Antiresorptive/immunotherapy in spine metastasis
Given the role that osteoclasts play in the reactivation and growth of tumor cells, medications which reduce osteoclastic activity can help control the progression of skeletal metastasis (28). A randomized control trial published in 2015 showed that adjuvant denosumab therapy in postmenopausal women with breast cancer reduced the number of pathologic fractures and delayed the time to the first clinical fracture (39). Another randomized control trial published by Saad et al. (40) showed that zoledronic acid reduces the risk of developing pathologic fractures in patients with hormone-refractory metastatic prostate carcinoma. A 2015 meta-analysis of the results of randomized control trials on adjuvant bisphosphonate therapy in breast cancer showed that bisphosphonates reduce the risk of cancer recurrence in the bone (41). Very recently, a systematic review indicated that use of bisphosphonates in management of metastatic disease to the bone was cost effective and resulted in lower mortality and improved quality of life for patients (42). In contrast, use of denosumab was found to be “marginally more effective” for improving outcomes than bisphosphonates (42). However, the high price of the drug resulted in a much higher cost for each quality-adjusted life year gained as compared to bisphosphonates, rendering denosumab treatment not cost effective (42).
Local vs. systemic treatment
Bisphosphonates and denosumab are both administered systemically, which has been associated with several negative side effects. High systemic doses of zoledronic acid were found to be associated with renal function deterioration (40). Other potential side effects include fevers, myalgia, hypocalcaemia, osteonecrosis of the jaw and atrial fibrillation (43). To circumvent these challenges, efforts are being made to explore effects of local bisphosphonate delivery at the site of boney metastases. We have previously assessed the efficacy of local vs. systemic delivery of zoledronic acid in a metastatic murine xenograft model (44). A metastatic tumor was established in the proximal tibia and each animal was treated with a weight adjusted dose of 0.025 mg/kg of zoledronic acid once a week delivered either systemically or locally. The animals were treated for a total of 4 weeks. We showed that mice which received locally administered zoledronate had a statistically significant 44.8% increase in bone volume/tissue volume % relative to those receiving systemic zoledronate (44). These results show that local delivery of zoledronate can improve local bone quality in the setting of bone metastasis. We also found that there was an increase in tumor cell apoptosis and a decrease in tumor cell proliferation, but neither of these findings reached statistical significance (44). While these results are impressive, they are likely to be challenging to implement in a clinical setting given the resources that would be needed to perform the weekly procedures. Therefore, development of drug delivery devices may be a good approach to local delivery of bisphosphonates or other agents such as denosumab antibodies.
Current advancements in tissue engineering and targeted drug delivery
Development of an optimal solution to address the shortcomings of current surgical management of patients with metastatic spine disease will require a multidisciplinary approach. As outlined earlier, a major focus for management of these patients is adequate viability of the graft used to fill the defect created after resection of a metastatic lesion from the vertebrae. Current treatment options have a limited ability in preventing tumor recurrence, promoting bone regeneration, and restoring the original structural integrity of the involved segments. Outlined below are recent biomedical advancements that explore novel solutions to limitations in surgical management of patients with metastatic spine disease.
Bone cements
Bone cement has been used extensively in surgery for more than half a century, with the first orthopedic application being performed by the English surgeon Dr. John Charnley for implant fixation in hip replacement operations (45). Use of cements in surgical management of metastatic spine disease allows for restoration of structural integrity, leading to an improved pain score and function in patients (46-49). Commonly used cements include calcium phosphate and polymethyl methacrylate (PMMA), with a variety of composite formulations with other compounds existing to achieve unique structural and chemical properties.
Calcium phosphate cement (CPC) is bio-resorbable (50,51), which can provide a scaffold within a defect for eventual bone regeneration. In its pure form, however, CPC has poor stress tolerance and is brittle, making it unsuitable for use in the weight-bearing spine (52,53). However, composite formulations of CPC have more favorable mechanical properties, as outlined by Hu et al. (54) in a recent study with the use of silk fibroin to reinforce the cement. Although CPC alone is a poor modality for sustained drug release (55), a very recent report demonstrated that conjugating CPC with polylactic-co-glycolic acid (PLGA) microspheres allows the compound to release 25% of loaded Alendronate over a 148-day period (55). Another study has shown that zoledronate impregnated calcium deficient apatite (CDA) was able to sustainably release the drug to inhibit osteoclast number by 85% and decrease osteoclastic bone resorption by 3.3-fold without hindering osteoblast function in an in vitro rabbit bone culture (56). Despite its limited use as a standalone bone substitute in the spine, CPC and its composite formulations carry high potential for sustained local delivery of therapeutics in spinal metastasis patients.
PMMA cement is widely used in vertebroplasty procedures. The ability of PMMA to create a mechanically stiff core inside the vertebrae make it ideal for filling structural voids within the spine (57). Unique antitumor properties of PMMA cement have also been proposed, with heat induced tumor necrosis from the high temperatures of cement curing (58), to direct cytotoxic effects of PMMA monomers on cells in proximity to the cement (59). Recent trends in drug therapy have also shown promising potential for PMMA as a tool for local drug delivery. Antibiotic impregnated PMMA cement is widely used in surgical procedures to provide sustained and local concentrations of a multitude of antibiotics, such as tobramycin, while minimizing systemic exposure to these drugs (60). Interestingly, a recent study investigated local delivery of zoledronate to treat bony malignant tumors (61). In this study, zoledronate was loaded into commercially available formulations of hydroxyapatite and polymethyl methacrylate (PMMA) bone cement. The cement containing zoledronate was found to decrease tumor cell viability. Unfortunately, these effects were not sustained over the 14-day course of the experiment (61). The authors also noted that although the zoledronate-hydroxyapatite combination did exhibit antitumor effects, these effects were weaker compared to the zoledronate-PMMA formulation. This difference was hypothesized to be due to higher affinity of zoledronate to hydroxyapatite (61). This study also assessed the impact of local zoledronate delivery on serum creatinine and blood urea nitrogen as surrogate measures of renal function, and all parameters remained within normal limits (61). While these implants were able to produce an antitumor effect, it was only sustained for 14 days (61). Given what we know about the process of bone metastasis, and the presence of dormant tumor cells within the bone, such a short duration is unlikely to be of a large clinical benefit and more research into this topic is warranted. Indeed, one group recently reported in a phase-1 clinical trial of 17 patients that local delivery of zoledronate through bone cement was safe, did not cause any side effects and may have reduced local recurrence of giant cell tumor of bone (62). It is important to note that a phase 2 randomized control clinical trial is currently underway by this same group at St. Louis University investigating whether 4 mg zoledronate mixed with PMMA cement can decrease the local recurrence rate of giant cell tumor of bone following curettage in 120 patients. All these reports indicate that local delivery of bisphosphonates within a structural carrier has high potential for blocking spine metastasis recurrence and stabilizing the bone following resection.
Nanoparticles
Nanotechnology has emerged over the past decades as a promising approach for targeted drug therapy. Nanoparticles (NP) allow for therapeutic control in dimensions never seen before in modern medicine (63). These versatile particles posses many different chemical and biophysical properties, making them very attractive for localized drug therapy in a multitude of diseases. They can function as drug sequestrants, prolonging the half life of drugs by protecting their degradation and elimination from the body (64). Through a phenomenon known as “Enhanced Permeability and Retention (EPR)” outlined by Matsumura et al. (65) in 1986, nanoparticles have been observed to passively target and accumulate in malignant tissue due to the increased permeability of their hypervascular environment and decreased lymphatic drainage. Additionally, preclinical animal studies of pH-responsive nanoparticles loaded with chemotherapeutics have demonstrated increased drug activity within acidic tumor environments when compared to pH-unresponsive nanoparticles or free drug administration (66,67).
Nanoparticles have been studied extensively as a viable option for sustained and local delivery of chemotherapeutics and antiresorptive medication, making them good candidates for study in metastatic spine disease. Doxorubicin-conjugated polyethylene glycol (PEG) nanoparticles have been investigated for intravenous treatment of primary and metastatic human osteosarcoma cell lines (68). This in vitro study demonstrated that the nanoparticle-conjugated Doxorubicin achieved the same levels of tumor cellular uptake at one tenth of the concentration of free Doxorubicin. This translated to a 40% greater inhibition of tumor growth when compared to systemic delivery of free drug in a mouse tumor model (68). Another study demonstrated the effectiveness of intra-tumoral delivery of Paclitaxel-conjugated hyaluronan nanoparticles in treatment of breast cancer cell lines (69), where the nanoparticle-conjugated Paclitaxel was able to achieve the same therapeutic effect as free drug. However, the in vivo intra-tumoral injection of nanoparticle-conjugated Paclitaxel surprisingly led to a 50% decrease in tumor size over 57 days as compared to an almost 5-fold increase in size in the free drug intratumor injection group (69). Furthermore, mesoporous silica nanoparticles in combination with PMMA cement are being studied for use in targeted and sustained drug delivery (70-72). Incorporation of drug-loaded nanoparticles into cement scaffolds filling large bone defects can have applications for surgical management of metastatic spine disease (Figure 1). These studies have showcased the superior ability of nanoparticles in extending and concentrating the therapeutic actions of drugs compared to treatment with free drug which is the standard of care in cancer therapy today.
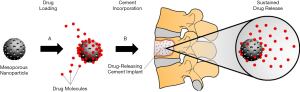
3D printing
With the advent of three-dimensional (3D) printing technology, also known as additive manufacturing, it has become possible to design and materialize complex objects without the need for sophisticated manufacturing equipment. With the aid of user-friendly computer design software, researchers can obtain and test any desired structure in a timely and cost-effective manner (73). A diverse variety of materials are possible to 3D print, including metals, ceramics, polymers, and hydrogels containing live cells for bioprinting. Some of the polymers used are FDA approved, such as polylactic acid, polycaprolactone and polyglycolic acid, which also can be designed to possess appropriate mechanical properties for orthopaedic applications.
The use of biocompatible and osteoinductive compounds in 3D printing has paved the way for novel approaches to bone regeneration and tissue engineering. A recent study by Heo et al. (74) demonstrated feasibility of coating osteoinductive fish bone extract on polycaprolactone 3D-printed scaffolds in an in vitro osteogenic model using a mouse pre-osteoblast cell line. Their results demonstrated that this treated construct increased calcium deposition onto the scaffold by more than 5-fold (74). A study by Hutmacher et al. (75) concluded that PCL 3D-printed scaffolds allow for continued proliferation and matrix production of human fibroblasts and periosteal cells over a 4-week period in an in vitro model. An in vivo sheep tibia model by Cipitria et al. (76) demonstrated that 3D-printed PCL scaffolds allow for retention and prolonging the effect of the growth factor bone morphogenetic protein (BMP), decreasing the need for administration of costly supraphysiological doses of the protein. These studies provide new insight into the versatility of 3D-printed biocompatible constructs in providing a scaffold for bone regeneration. Several studies have indicated feasibility of using 3D-printed ceramics as maxillofacial bone substitutes (77-79). These types of scaffolds could also presumably be used as drug delivery devices.
In addition to tissue regeneration and repair, 3D-printed scaffolds have been shown to be effective at delivering drugs locally in a sustainable manner. Our laboratory has recently demonstrated sustained Doxorubicin delivery in an in vitro 2D prostate cancer model using a novel nanoporous PORO-LAY 3D-printed scaffold (80). The PORO-LAY polymer is a thermoplastic polyurethane (TPU) and polyvinyl alcohol (PVA) co-polymer (81). This polymer is unique in that it can be 3D-printed into any desired shape as a rigid plastic. However, the PVA component dissolves upon washing the construct with water, transforming it into a sponge riddled with drug-absorbent nanopores (80). We demonstrated that doxorubicin delivery from the nanoporous scaffold was able to achieve roughly 60% reduction in metabolic activity of patient-derived prostate cancer spine metastasis cells. This was comparable to the same reductions in metabolic activity observed with direct treatment of the cells with Doxorubicin (80). A follow up study testing the effectiveness of these scaffolds for targeted delivery of bisphosphonates is currently underway.
Bioprinting
Three-dimensional printing has revolutionized our capacity for creating physical objects, and the same principle has great potential for creating complex tissue-like structures composed of living cells and other biomaterials that closely mimic in vivo microenvironments. Advancing technology, free access to design software and reduction in overall cost of bioprinter hardware has allowed for creation of sophisticated machines that can handle and seed cells in a safe and precise manner (82-85). There are several types of bioprinting techniques, but the most widely used involves suspending cells inside an extracellular matrix-like bioink material and extruding layers one on top of the next. Natural bioinks commonly consist of collagen (86), alginate (87), chitosan (88), and silk fibroin (89). Synthetic bioinks consist of PCL (90), polyethylene glycol (PEG) (91), and hydroxyapatite (92), which are suitable for bioprinting more rigid models for studying cartilage and bone (91). Current applications of bioprinting involve creating testing models for various tissue types such as skin (93), cardiovascular (94-96) and bone (97). Regarding bone, bioprinting is effectively combined with 3D printing to create a rigid scaffold with subsequent seeding of osteoprogenitor cells within the scaffold. Addition of growth factors or defined osteogenic medium into the bioink will provide additional stimulation for progenitor cells to undergo osteogenic differentiation within the construct (98).
Bioprinting can be an effective tool for studying metastatic spine disease through creation of tumor models that more accurately study tumor behavior in a three-dimensional tissue-like environment as opposed to current 2D cultures (85,99). Cells behave and respond to therapeutics differently in a 3D environment as opposed to the 2D environment of conventional cell culture techniques (100,101). One area of interest is drug sensitivity of tumors when they are arranged as 3D spheroids. A study by Zhao et al. (100) showcased how tumor spheroids exhibited greater resistance to chemotherapeutics than 2D models. The presence of stromal cells in the microenvironment can greatly influence tumor growth and progression. A study by Zhou et al. studied the effects of co-culturing human breast cancer cells and osteoblasts in a bioprinted 3D model. They observed increased breast cancer proliferation and a reduction in proliferation of osteoblast cells. Additionally, breast cancer cell secretion of vascular endothelial growth factor (VEGF) was increased while alkaline phosphatase (ALP) secretion by osteoblast cells was decreased. Bioprinting provided the advantage for precise placement of cells in separate, specific compartments that allowed for cell-cell communication and analysis of cell proliferation in three-dimensional space (101). Hence, bioprinting can allow for creation of complex three-dimensional tumor models that mimic the in vivo bone environment. This provides more clinically relevant testing of therapeutics and cell-cell interactions, serving as an offshoot for subsequent animal studies.
Animal models
Animal studies are an important initial step in assessing the clinical applicability of in vitro studies. Promising in vitro results must be confirmed within an in vivo environment to account for variables such as toxicity, immune response and pharmacokinetics. Animal models have been extensively studied with tissue-engineered constructs that promote in vivo bone regeneration (102-104). These constructs can be a combination of 3D-printed scaffolds, osteoprogenitor cells and growth factors (103,105-107). Common osteoconductive materials used in these scaffolds are hydroxyapatite, PCL, coral and ceramics, which contain a nanoporous structure ideal for host cell invasion (102,108-112). Additionally, different animal species provide certain advantages for studying bone regeneration. Smaller animals, such as mice, are suitable for studying ectopic bone formation (105). Due to their more similar size and mechanical loading characteristics as compared to humans, large animals, such as pigs and sheep, are suitable for studying tissue regeneration within bone defects through utilization of engineered constructs (105).
Large constructs designed to fill critical size bone defects are limited in their capability for widespread bone regeneration due to inadequate angiogenesis throughout their structure (113). Sathy et al. (102) used a multilayered construct design with alternating layers of osteoconductive PCL and calcium phosphate ceramic with angiogenic collagen/fibronectin zones. These zones allowed for through-the-thickness vessel formation inside the scaffold, resulting in widespread tissue regeneration inside the construct in a mouse model. Another approach for scaffold vascularization is providing an axial blood supply by incorporating the scaffold around an existing blood vessel (103,104,114). A study by Zimmerer et al. (104) vascularized a hollow beta-tricalcium phosphate scaffold using the thoracodorsal trunk of sheep. They observed that over a 6-month period, the scaffold had transformed into a solid bioartificial bone graft with widespread vascularization. Another study by Kaempfen et al. (113) vascularized decellularized trabecular bone cylinders with an axial blood supply from a branch of the axillary artery in rabbits. They compared widespread vascularization and bone formation between scaffolds that were ectopically incubated for 6 weeks before implantation into a segmental humerus defect and vascularized scaffolds that were implanted without incubation. Their results showed more vascularization throughout the incubated implant compared to implantation without prior incubation (113). However, the degree of bone formation they observed in both implants was minimal which they attributed to local inflammation around the bone defect.
In addition to these in vivo studies that showcase the ability of tissue-engineered constructs for bone repair, several in vivo human xenograft (115) and, more recently, patient-derived xenograft animal models (116) of various cancer types exist. The premise of these models is to implant human cancer cells from established/characterized cell lines or patient-derived tumor cells either subcutaneously or in the bone of these animals for example. Next, the animals can be treated with novel systemic therapeutics, nanoparticle carriers or implantable constructs following tumor resection. Most recently, efforts to model the human immune response to cancer have been made by “humanizing” immunocompromised animals through implantation of human immune cells within their bone marrow. A study by Shafiee et al. (117) humanized immunodeficient mice through inoculation of their bone marrow with human CD34+ cells. Upon xenografting human breast cancer cells, they observed that the humanized mice showed less tumor burden and metastasis compared to immunodeficient mice. These types of animal models can allow for addressing feasibility and optimal dosage requirements for novel therapeutics and carriers of therapeutics by creating animal disease models that more closely resemble human tissue.
Several studies have successfully shown how intravertebral tumor models can be used reliably to study neurological deterioration in animals (118-122). A study by Tatsui et al. (118) demonstrated how L-3 vertebral human lung cancer xenografts in mice can lead to paraplegia over 30 days. Their histological findings highly correlated with motor function assessment of these mice over the course of the study (118). Studies such as this are highly suitable for modelling spine metastasis. However, no studies to date have used such a spine metastasis model to study therapeutic interventions as most models focus on long bones of animals (44). Nonetheless, resection in the mouse/rat spine followed by implantation with bone substitutes or 3D printed constructs will be far more difficult than in the long bone of these animals. Although more costly, rabbits or larger animals may provide a larger anatomical site (123) in which to perform resection of induced tumors. This will be more feasible for implantation of biomaterials for bone repair and anti-cancer treatment. However, generating immune deficient rabbits or other large animals will be costly and not mainstream, which limits their potential xenograft studies (124). Advancing the rodent spine metastasis models outlined in this review is likely the most cost-effective way to study tissue engineering strategies to treat spine metastasis.
Future directions
Despite the exciting advancements in the fields of targeted drug therapy and tissue engineering to treat and repair resected spinal metastases, limited human trial data are available to assess the true clinical applicability of these innovations. Personalized treatment of resected metastatic spine tumors using 3D printing technology appears most promising. Indeed, an ongoing clinical trial at Southern Medical University in China is applying 3D-printed implants to bone defects during metastatic spine disease treatment (125). Amazingly, this trial is a multicentered randomized controlled study including 300 participants, which is scheduled to be completed by December of 2021 (125). If 3D-printed constructs as a bone substitute are found to be a valid solution, this may prompt further consideration by regulatory bodies such as the FDA. These new insights further support the idea of using 3D printing in personalized treatment for spine metastases in the near future.
Conclusions
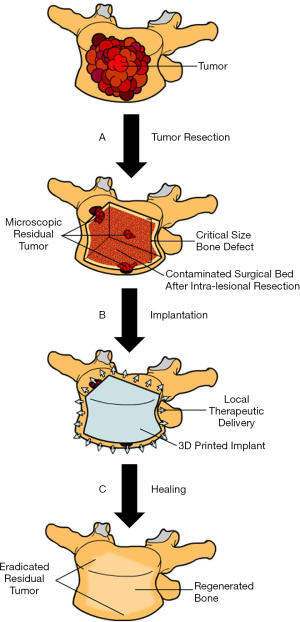
Improved quality of life continues to be an important goal for managing patients with metastatic spine disease. The studies outlined in this review have demonstrated how tissue engineering is being used for bone repair and regeneration. Tissue-engineered bone substitutes can also circumvent the limitations associated with bone grafting, such as donor site complications and limited supply. Furthermore, these novel bone substitutes can stabilize large bone defects and deliver chemotherapeutics locally to inhibit cancer recurrence and minimize toxicity associated with systemic drug delivery. Many of the bone substitutes outlined in this review possess capacity to deliver therapeutics, with antibiotic impregnated PMMA being the most clinically relevant. By combining recent advancements in tissue engineering and targeted therapy, new approaches to stabilize large bone defects, promote bone regeneration and locally deliver a specific cocktail of therapeutics is within reach (Figure 2). There is a great opportunity to achieve this goal; however, multidisciplinary strategies combining basic science, engineering and clinical principles must continue to be applied in future work.
Acknowledgments
Funding: This work was performed with the support of an AO Start Up grant S-16-138W to MH Weber. P Ahangar received support from a MITACs Accelerate Grant to MH Weber. M Aziz received support from the Reseau de Recherche en Sante Buccodentaire et Osseuse (RSBO).
Footnote
Conflicts of Interest: The authors have no conflict of interest to declare.
References
- Aaron AD. The management of cancer metastatic to bone. JAMA 1994;272:1206-9. [Crossref] [PubMed]
- Sciubba DM, Petteys RJ, Dekutoski MB, et al. Diagnosis and management of metastatic spine disease. A review. J Neurosurg Spine 2010;13:94-108. [Crossref] [PubMed]
- Batista N, Tee J, Sciubba D, et al. Emerging and established clinical, histopathological and molecular parametric prognostic factors for metastatic spine disease secondary to lung cancer: Helping surgeons make decisions. J Clin Neurosci 2016;34:15-22. [Crossref] [PubMed]
- Clarke MJ, Molina CA, Fourney DR, et al. Systematic Review of the Outcomes of Surgical Treatment of Prostate Metastases to the Spine. Global Spine J 2017;7:460-8. [Crossref] [PubMed]
- Sciubba DM, Goodwin CR, Yurter A, et al. A Systematic Review of Clinical Outcomes and Prognostic Factors for Patients Undergoing Surgery for Spinal Metastases Secondary to Breast Cancer. Global Spine J 2016;6:482-96. [Crossref] [PubMed]
- Goodwin CR, Clarke MJ, Gokaslan ZL, et al. En Bloc Resection of Solitary Functional Secreting Spinal Metastasis. Global Spine J 2016;6:277-83. [Crossref] [PubMed]
- CCSA. Canadian Cancer Statistics 2018. Toronto, ON, Canada 2018.
- Barzilai O, Versteeg AL, Sahgal A, et al. Survival, local control, and health-related quality of life in patients with oligometastatic and polymetastatic spinal tumors: A multicenter, international study. Cancer 2019;125:770-8. [Crossref] [PubMed]
- Versteeg AL, Sahgal A, Rhines LD, et al. Psychometric evaluation and adaptation of the Spine Oncology Study Group Outcomes Questionnaire to evaluate health-related quality of life in patients with spinal metastases. Cancer 2018;124:1828-38. [Crossref] [PubMed]
- Aoude A, Fortin M, Aldebeyan S, et al. The revised Tokuhashi score; analysis of parameters and assessment of its accuracy in determining survival in patients afflicted with spinal metastasis. Eur Spine J 2018;27:835-40. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005;366:643-8. [Crossref] [PubMed]
- Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976) 2010;35:E1221-9. [Crossref] [PubMed]
- Fisher CG, Schouten R, Versteeg AL, et al. Reliability of the Spinal Instability Neoplastic Score (SINS) among radiation oncologists: an assessment of instability secondary to spinal metastases. Radiat Oncol 2014;9:69. [Crossref] [PubMed]
- Arana E, Kovacs FM, Royuela A, et al. Spine Instability Neoplastic Score: agreement across different medical and surgical specialties. Spine J 2016;16:591-9. [Crossref] [PubMed]
- Dunning EC, Butler JS, Morris S. Complications in the management of metastatic spinal disease. World J Orthop 2012;3:114-21. [Crossref] [PubMed]
- Altaf F, Weber M, Dea N, et al. Evidence-Based Review and Survey of Expert Opinion of Reconstruction of Metastatic Spine Tumors. Spine (Phila Pa 1976) 2016;41 Suppl 20:S254-61. [Crossref] [PubMed]
- Abu-Bonsrah N, Goodwin CR, De la Garza-Ramos R, et al. Readmissions After Surgical Resection of Metastatic Tumors of the Spine at a Single Institution. World Neurosurgery 2017;101:695-701.e1. [Crossref] [PubMed]
- Quraishi NA, Rajabian A, Spencer A, et al. Reoperation rates in the surgical treatment of spinal metastases. Spine J 2015;15:S37-43. [Crossref] [PubMed]
- Lau D, Chan AK, Theologis AA, et al. Costs and readmission rates for the resection of primary and metastatic spinal tumors: a comparative analysis of 181 patients. J Neurosurg Spine 2016;25:366-78. [Crossref] [PubMed]
- Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 2003;61:1115-7. [Crossref] [PubMed]
- Perazella MA, Markowitz GS. Bisphosphonate nephrotoxicity. Kidney Int 2008;74:1385-93. [Crossref] [PubMed]
- Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer 2003;3:453-8. [Crossref] [PubMed]
- Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 1989;8:98-101. [PubMed]
- Hart IR, Fidler IJ. Role of organ selectivity in the determination of metastatic patterns of B16 melanoma. Cancer Res 1980;40:2281-7. [PubMed]
- Fidler IJ. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2'-deoxyuridine. J Natl Cancer Inst 1970;45:773-82. [PubMed]
- Fidler IJ, Gruys E, Cifone MA, et al. Demonstration of multiple phenotypic diversity in a murine melanoma of recent origin. J Natl Cancer Inst 1981;67:947-56. [PubMed]
- Fidler IJ, Talmadge JE. Evidence that intravenously derived murine pulmonary melanoma metastases can originate from the expansion of a single tumor cell. Cancer Res 1986;46:5167-71. [PubMed]
- Croucher PI, McDonald MM, Martin TJ. Bone metastasis: the importance of the neighbourhood. Nat Rev Cancer 2016;16:373-86. [Crossref] [PubMed]
- Ruppender NS, Merkel AR, Martin TJ, et al. Matrix rigidity induces osteolytic gene expression of metastatic breast cancer cells. PLoS One 2010;5:e15451. [Crossref] [PubMed]
- Page JM, Merkel AR, Ruppender NS, et al. Matrix rigidity regulates the transition of tumor cells to a bone-destructive phenotype through integrin beta3 and TGF-beta receptor type II. Biomaterials 2015;64:33-44. [Crossref] [PubMed]
- Johnson RW, Merkel AR, Danilin S, et al. 6-Thioguanine inhibition of parathyroid hormone-related protein expression is mediated by GLI2. Anticancer Res 2011;31:2705-12. [PubMed]
- Johnson RW, Nguyen MP, Padalecki SS, et al. TGF-beta promotion of Gli2-induced expression of parathyroid hormone-related protein, an important osteolytic factor in bone metastasis, is independent of canonical Hedgehog signaling. Cancer Res 2011;71:822-31. [Crossref] [PubMed]
- Gallwitz WE, Guise TA, Mundy GR. Guanosine nucleotides inhibit different syndromes of PTHrP excess caused by human cancers in vivo. J Clin Invest 2002;110:1559-72. [Crossref] [PubMed]
- Ottewell PD, Wang N, Brown HK, et al. Zoledronic acid has differential antitumor activity in the pre- and postmenopausal bone microenvironment in vivo. Clin Cancer Res 2014;20:2922-32. [Crossref] [PubMed]
- Ottewell PD, Wang N, Meek J, et al. Castration-induced bone loss triggers growth of disseminated prostate cancer cells in bone. Endocr Relat Cancer 2014;21:769-81. [Crossref] [PubMed]
- Domschke C, Diel IJ, Englert S, et al. Prognostic value of disseminated tumor cells in the bone marrow of patients with operable primary breast cancer: a long-term follow-up study. Ann Surg Oncol 2013;20:1865-71. [Crossref] [PubMed]
- Chen Z, Orlowski RZ, Wang M, et al. Osteoblastic niche supports the growth of quiescent multiple myeloma cells. Blood 2014;123:2204-8. [Crossref] [PubMed]
- Lawson MA, McDonald MM, Kovacic N, et al. Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nat Commun 2015;6:8983. [Crossref] [PubMed]
- Gnant M, Pfeiler G, Dubsky PC, et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2015;386:433-43. [Crossref] [PubMed]
- Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 2002;94:1458-68. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative G. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 2015;386:1353-61. [Crossref] [PubMed]
- Andronis L, Goranitis I, Bayliss S, et al. Cost-Effectiveness of Treatments for the Management of Bone Metastases: A Systematic Literature Review. Pharmacoeconomics 2018;36:301-22. [Crossref] [PubMed]
- Coleman R, Burkinshaw R, Winter M, et al. Zoledronic acid. Expert Opin Drug Saf 2011;10:133-45. [Crossref] [PubMed]
- Nooh A, Zhang YL, Sato D, et al. Intra-tumor delivery of zoledronate mitigates metastasis-induced osteolysis superior to systemic administration. J Bone Oncol 2017;6:8-15. [Crossref] [PubMed]
- Charnley J. The classic: The bonding of prostheses to bone by cement. 1964. Clin Orthop Relat Res 2010;468:3149-59. [Crossref] [PubMed]
- Floeth FW, Herdmann J, Rhee S, et al. Open microsurgical tumor excavation and vertebroplasty for metastatic destruction of the second cervical vertebra-outcome in seven cases. Spine J 2014;14:3030-7. [Crossref] [PubMed]
- Zhao XJ, Qi XS, Mao ZX, et al. Zhongguo Gu Shang 2017;30:115-20. [Percutaneous vertebroplasty and open vertebroplasty for metastatic spinal tumor]. [PubMed]
- Bao L, Jia P, Li J, et al. Percutaneous Vertebroplasty Relieves Pain in Cervical Spine Metastases. Pain Res Manag 2017;2017:3926318. [Crossref] [PubMed]
- McGraw JK, Lippert JA, Minkus KD, et al. Prospective evaluation of pain relief in 100 patients undergoing percutaneous vertebroplasty: results and follow-up. J Vasc Interv Radiol 2002;13:883-6. [Crossref] [PubMed]
- Yuan H, Li Y, de Bruijn JD, et al. Tissue responses of calcium phosphate cement: a study in dogs. Biomaterials 2000;21:1283-90. [Crossref] [PubMed]
- Yang J, Zhang K, Zhang S, et al. Preparation of calcium phosphate cement and polymethyl methacrylate for biological composite bone cements. Med Sci Monit 2015;21:1162-72. [Crossref] [PubMed]
- Chain M LW, Lemons JE, et al. Mechanical behavior and the dissolution characteristics of a calcium phosphate cement for bone replacement: University of Alabama at Birmingham, 1997.
- Ishikawa K, Asaoka K. Estimation of ideal mechanical strength and critical porosity of calcium phosphate cement. J Biomed Mater Res 1995;29:1537-43. [Crossref] [PubMed]
- Hu M, He Z, Han F, et al. Reinforcement of calcium phosphate cement using alkaline-treated silk fibroin. Int J Nanomedicine 2018;13:7183-93. [Crossref] [PubMed]
- van Houdt CIA, Gabbai-Armelin PR, Lopez-Perez PM, et al. Alendronate release from calcium phosphate cement for bone regeneration in osteoporotic conditions. Sci Rep 2018;8:15398. [Crossref] [PubMed]
- Faucheux C, Verron E, Soueidan A, et al. Controlled release of bisphosphonate from a calcium phosphate biomaterial inhibits osteoclastic resorption in vitro. J Biomed Mater Res A 2009;89:46-56. [Crossref] [PubMed]
- Boger A, Bisig A, Bohner M, et al. Variation of the mechanical properties of PMMA to suit osteoporotic cancellous bone. J Biomater Sci Polym Ed 2008;19:1125-42. [Crossref] [PubMed]
- San Millan Ruiz D, Burkhardt K, Jean B, et al. Pathology findings with acrylic implants. Bone 1999;25:85S-90S. [Crossref] [PubMed]
- Dahl OE, Garvik LJ, Lyberg T. Toxic effects of methylmethacrylate monomer on leukocytes and endothelial cells in vitro. Acta Orthop Scand 1994;65:147-53. [Crossref] [PubMed]
- Josefsson G, Gudmundsson G, Kolmert L, et al. Prophylaxis with systemic antibiotics versus gentamicin bone cement in total hip arthroplasty. A five-year survey of 1688 hips. Clin Orthop Relat Res 1990.173-8. [PubMed]
- Koto K, Murata H, Sawai Y, et al. Cytotoxic effects of zoledronic acid-loaded hydroxyapatite and bone cement in malignant tumors. Oncol Lett 2017;14:1648-56. [Crossref] [PubMed]
- Greenberg DD, Lee FY. Bisphosphonate-loaded Bone Cement as a Local Adjuvant Therapy for Giant Cell Tumor of Bone: A 1 to 12-Year Follow-up Study. Am J Clin Oncol 2019;42:231-7. [Crossref] [PubMed]
- Kopp M, Kollenda S, Epple M. Nanoparticle-Protein Interactions: Therapeutic Approaches and Supramolecular Chemistry. Acc Chem Res 2017;50:1383-90. [Crossref] [PubMed]
- Shalgunov V, Zaytseva-Zotova D, Zintchenko A, et al. Comprehensive study of the drug delivery properties of poly(l-lactide)-poly(ethylene glycol) nanoparticles in rats and tumor-bearing mice. J Control Release 2017;261:31-42. [Crossref] [PubMed]
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 1986;46:6387-92. [PubMed]
- Kang Y, Ha W, Liu YQ, et al. pH-responsive polymer-drug conjugates as multifunctional micelles for cancer-drug delivery. Nanotechnology 2014;25:335101. [Crossref] [PubMed]
- Hamaguchi T, Matsumura Y, Suzuki M, et al. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br J Cancer 2005;92:1240-6. [Crossref] [PubMed]
- Rudnick-Glick S, Corem-Salkmon E, Grinberg I, et al. Targeted drug delivery of near IR fluorescent doxorubicin-conjugated poly(ethylene glycol) bisphosphonate nanoparticles for diagnosis and therapy of primary and metastatic bone cancer in a mouse model. J Nanobiotechnology 2016;14:80. [Crossref] [PubMed]
- Al-Ghananeem AM, Malkawi AH, Muammer YM, et al. Intratumoral delivery of Paclitaxel in solid tumor from biodegradable hyaluronan nanoparticle formulations. AAPS PharmSciTech 2009;10:410-7. [Crossref] [PubMed]
- Shen SC, Ng WK, Shi Z, et al. Mesoporous silica nanoparticle-functionalized poly(methyl methacrylate)-based bone cement for effective antibiotics delivery. J Mater Sci Mater Med 2011;22:2283-92. [Crossref] [PubMed]
- Slowing II, Vivero-Escoto JL, Wu CW, et al. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliv Rev 2008;60:1278-88. [Crossref] [PubMed]
- Mekaru H, Lu J, Tamanoi F. Development of mesoporous silica-based nanoparticles with controlled release capability for cancer therapy. Adv Drug Deliv Rev 2015;95:40-9. [Crossref] [PubMed]
- Rosenzweig DH, Carelli E, Steffen T, et al. 3D-Printed ABS and PLA Scaffolds for Cartilage and Nucleus Pulposus Tissue Regeneration. Int J Mol Sci 2015;16:15118-35. [Crossref] [PubMed]
- Heo SY, Ko SC, Oh GW, et al. Fabrication and characterization of the 3D-printed polycaprolactone/fish bone extract scaffolds for bone tissue regeneration. J Biomed Mater Res B Appl Biomater 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Hutmacher DW, Schantz T, Zein I, et al. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J Biomed Mater Res 2001;55:203-16. [Crossref] [PubMed]
- Cipitria A, Reichert JC, Epari DR, et al. Polycaprolactone scaffold and reduced rhBMP-7 dose for the regeneration of critical-sized defects in sheep tibiae. Biomaterials 2013;34:9960-8. [Crossref] [PubMed]
- Gbureck U, Vorndran E, Muller FA, et al. Low temperature direct 3D printed bioceramics and biocomposites as drug release matrices. J Control Release 2007;122:173-80. [Crossref] [PubMed]
- Habibovic P, Gbureck U, Doillon CJ, et al. Osteoconduction and osteoinduction of low-temperature 3D printed bioceramic implants. Biomaterials 2008;29:944-53. [Crossref] [PubMed]
- Tamimi F, Torres J, Gbureck U, et al. Craniofacial vertical bone augmentation: a comparison between 3D printed monolithic monetite blocks and autologous onlay grafts in the rabbit. Biomaterials 2009;30:6318-26. [Crossref] [PubMed]
- Ahangar P, Akoury E, Ramirez Garcia Luna AS, et al. Nanoporous 3D-Printed Scaffolds for Local Doxorubicin Delivery in Bone Metastases Secondary to Prostate Cancer. Materials (Basel) 2018;11. [Crossref] [PubMed]
- Belka M, Ulenberg S, Baczek T. Fused Deposition Modeling Enables the Low-Cost Fabrication of Porous, Customized-Shape Sorbents for Small-Molecule Extraction. Analytical Chemistry 2017;89:4373-6. [Crossref] [PubMed]
- Knowlton S, Anand S, Shah T, et al. Bioprinting for Neural Tissue Engineering. Trends Neurosci 2018;41:31-46. [Crossref] [PubMed]
- Ma X, Liu J, Zhu W, et al. 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling. Adv Drug Deliv Rev 2018;132:235-51. [Crossref] [PubMed]
- Moore CA, Shah NN, Smith CP, et al. 3D Bioprinting and Stem Cells. Methods Mol Biol 2018;1842:93-103. [Crossref] [PubMed]
- Zhang YS, Duchamp M, Oklu R, et al. Bioprinting the Cancer Microenvironment. ACS Biomater Sci Eng 2016;2:1710-21. [Crossref] [PubMed]
- Yeo M, Lee JS, Chun W, et al. An Innovative Collagen-Based Cell-Printing Method for Obtaining Human Adipose Stem Cell-Laden Structures Consisting of Core-Sheath Structures for Tissue Engineering. Biomacromolecules 2016;17:1365-75. [Crossref] [PubMed]
- Axpe E, Oyen ML. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int J Mol Sci 2016;17. [Crossref] [PubMed]
- Demirtas TT, Irmak G, Gumusderelioglu M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017;9:035003. [Crossref] [PubMed]
- Rodriguez MJ, Brown J, Giordano J, et al. Silk based bioinks for soft tissue reconstruction using 3-dimensional (3D) printing with in vitro and in vivo assessments. Biomaterials 2017;117:105-15. [Crossref] [PubMed]
- Park SY, Choi JW, Park JK, et al. Tissue-engineered artificial oesophagus patch using three-dimensionally printed polycaprolactone with mesenchymal stem cells: a preliminary report. Interact Cardiovasc Thorac Surg 2016;22:712-7. [Crossref] [PubMed]
- Gao G, Hubbell K, Schilling AF, et al. Bioprinting Cartilage Tissue from Mesenchymal Stem Cells and PEG Hydrogel. Methods Mol Biol 2017;1612:391-8. [Crossref] [PubMed]
- Loebel C, Rodell CB, Chen MH, et al. Shear-thinning and self-healing hydrogels as injectable therapeutics and for 3D-printing. Nat Protoc 2017;12:1521-41. [Crossref] [PubMed]
- Lee V, Singh G, Trasatti JP, et al. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng Part C Methods 2014;20:473-84. [Crossref] [PubMed]
- Jana S, Lerman A. Bioprinting a cardiac valve. Biotechnol Adv 2015;33:1503-21. [Crossref] [PubMed]
- Norotte C, Marga FS, Niklason LE, et al. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009;30:5910-7. [Crossref] [PubMed]
- Gao L, Kupfer ME, Jung JP, et al. Myocardial Tissue Engineering With Cells Derived From Human-Induced Pluripotent Stem Cells and a Native-Like, High-Resolution, 3-Dimensionally Printed Scaffold. Circ Res 2017;120:1318-25. [Crossref] [PubMed]
- Fedorovich NE, Wijnberg HM, Dhert WJ, et al. Distinct tissue formation by heterogeneous printing of osteo- and endothelial progenitor cells. Tissue Eng Part A 2011;17:2113-21. [Crossref] [PubMed]
- Lee VK, Dai G. Printing of Three-Dimensional Tissue Analogs for Regenerative Medicine. Ann Biomed Eng 2017;45:115-31. [Crossref] [PubMed]
- Jiang T, Munguia-Lopez JG, Flores-Torres S, et al. Directing the Self-assembly of Tumour Spheroids by Bioprinting Cellular Heterogeneous Models within Alginate/Gelatin Hydrogels. Sci Rep 2017;7:4575. [Crossref] [PubMed]
- Zhao Y, Yao R, Ouyang L, et al. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 2014;6:035001. [Crossref] [PubMed]
- Zhou X, Zhu W, Nowicki M, et al. 3D Bioprinting a Cell-Laden Bone Matrix for Breast Cancer Metastasis Study. ACS Appl Mater Interfaces 2016;8:30017-26. [Crossref] [PubMed]
- Sathy BN, Mony U, Menon D, et al. Bone Tissue Engineering with Multilayered Scaffolds-Part I: An Approach for Vascularizing Engineered Constructs In Vivo. Tissue Eng Part A 2015;21:2480-94. [Crossref] [PubMed]
- Terheyden H, Jepsen S, Rueger DR. Mandibular reconstruction in miniature pigs with prefabricated vascularized bone grafts using recombinant human osteogenic protein-1: a preliminary study. Int J Oral Maxillofac Surg 1999;28:461-3. [Crossref] [PubMed]
- Zimmerer RM, Jehn P, Kokemuller H, et al. In vivo tissue engineered bone versus autologous bone: stability and structure. Int J Oral Maxillofac Surg 2017;46:385-93. [Crossref] [PubMed]
- McGovern JA, Griffin M, Hutmacher DW. Animal models for bone tissue engineering and modelling disease. Dis Model Mech 2018. [Crossref] [PubMed]
- Hofmann S, Hilbe M, Fajardo RJ, et al. Remodeling of tissue-engineered bone structures in vivo. Eur J Pharm Biopharm 2013;85:119-29. [Crossref] [PubMed]
- Hartman EH, Vehof JW, Spauwen PH, et al. Ectopic bone formation in rats: the importance of the carrier. Biomaterials 2005;26:1829-35. [Crossref] [PubMed]
- Mastrogiacomo M, Corsi A, Francioso E, et al. Reconstruction of extensive long bone defects in sheep using resorbable bioceramics based on silicon stabilized tricalcium phosphate. Tissue Eng 2006;12:1261-73. [Crossref] [PubMed]
- Mastrogiacomo M, Papadimitropoulos A, Cedola A, et al. Engineering of bone using bone marrow stromal cells and a silicon-stabilized tricalcium phosphate bioceramic: evidence for a coupling between bone formation and scaffold resorption. Biomaterials 2007;28:1376-84. [Crossref] [PubMed]
- Marcacci M, Kon E, Zaffagnini S, et al. Reconstruction of extensive long-bone defects in sheep using porous hydroxyapatite sponges. Calcif Tissue Int 1999;64:83-90. [Crossref] [PubMed]
- Manassero M, Viateau V, Deschepper M, et al. Bone regeneration in sheep using acropora coral, a natural resorbable scaffold, and autologous mesenchymal stem cells. Tissue Eng Part A 2013;19:1554-63. [Crossref] [PubMed]
- Decambron A, Manassero M, Bensidhoum M, et al. A comparative study of tissue-engineered constructs from Acropora and Porites coral in a large animal bone defect model. Bone Joint Res 2017;6:208-15. [Crossref] [PubMed]
- Kaempfen A, Todorov A, Guven S, et al. Engraftment of Prevascularized, Tissue Engineered Constructs in a Novel Rabbit Segmental Bone Defect Model. Int J Mol Sci 2015;16:12616-30. [Crossref] [PubMed]
- Weigand A, Beier JP, Hess A, et al. Acceleration of vascularized bone tissue-engineered constructs in a large animal model combining intrinsic and extrinsic vascularization. Tissue Eng Part A 2015;21:1680-94. [Crossref] [PubMed]
- Sausville EA, Burger AM. Contributions of human tumor xenografts to anticancer drug development. Cancer Res 2006;66:3351-4, discussion 3354. [Crossref] [PubMed]
- Yada E, Wada S, Yoshida S, et al. Use of patient-derived xenograft mouse models in cancer research and treatment. Future Sci OA 2017;4:FSO271. [Crossref] [PubMed]
- Shafiee A, McGovern JA, Lahr CA, et al. Immune system augmentation via humanization using stem/progenitor cells and bioengineering in a breast cancer model study. Int J Cancer 2018;143:1470-82. [Crossref] [PubMed]
- Tatsui CE, Lang FF, Gumin J, et al. An orthotopic murine model of human spinal metastasis: histological and functional correlations. J Neurosurg Spine 2009;10:501-12. [Crossref] [PubMed]
- Takahashi M, Ogawa J, Kinoshita Y, et al. Experimental study of paraplegia caused by spinal tumors: an animal model of spinal tumors created by transplantation of VX2 carcinoma. Spine J 2004;4:675-80. [Crossref] [PubMed]
- Arguello F, Baggs RB, Duerst RE, et al. Pathogenesis of vertebral metastasis and epidural spinal cord compression. Cancer 1990;65:98-106. [Crossref] [PubMed]
- Mantha A, Legnani FG, Bagley CA, et al. A novel rat model for the study of intraosseous metastatic spine cancer. J Neurosurg Spine 2005;2:303-7. [Crossref] [PubMed]
- Zhuang Y, Zhao W, Zhang W, et al. A reproducible model of intramedullary spinal cord tumor in rats bearing RG2 cells. Oncotarget 2017;8:30971-7. [Crossref] [PubMed]
- Amundson E, Pradilla G, Brastianos P, et al. A novel intravertebral tumor model in rabbits. Neurosurgery 2005;57:341-6; discussion 341-6. [Crossref] [PubMed]
- Kang C, Brennan JA, Kuzmiak-Glancy S, et al. Technical advances in studying cardiac electrophysiology - Role of rabbit models. Prog Biophys Mol Biol 2016;121:97-109. [Crossref] [PubMed]
- Clinical Application of Personal Designed 3D Printing Implants in Bone Defect Restoration. Available online: https://ClinicalTrials.gov/show/NCT03166917.


