Clinical presentation, management and outcomes of sacral metastases: a multicenter, retrospective cohort study
Introduction
Symptomatic spinal metastases occur in approximately 10–20% of the cancer population with the minority occurring in the sacrum (1,2). Preservation of neurological and physical function as well as addressing mechanical instability is primary objectives in the treatment of spinal metastases. For sacral metastases, a paucity of literature, unfamiliarity to most surgeons, and a historically high rate of adverse events (AEs) have led surgeons to shy away from operating on this population (3).
With the evolution of better surgical implants, less invasive procedures, intraoperative navigation, better blood salvage options and novel methods of radiation therapy (RT), the spinal surgeon is better equipped to effectively treat these patients. The impact of surgery and/or RT for sacral metastases on health related-quality of life (HRQOL) remains largely unknown. The primary objective of this study is to determine HRQOL and pain for patients with sacral metastases treated with surgery and/or RT. Secondary objectives were to describe the AE profile following RT or surgery and to observe how treatment affects neurologic function (lower extremity motor score, bowel and bladder function) in this population.
Methods
Design
Data were obtained from the Epidemiology, Process and Outcomes of Spine Oncology (EPOSO) [ClinicalTrials.gov (NCT01825161)], a prospective multicenter observational study on Spinal Metastases. This study was developed and funded by the AOSpine Knowledge Forum Tumor (AOSKFT) and by an Orthopedic Research and Education Foundation grant. Patients were recruited across 10 participating centers (North America and Europe) selected for their experience in handling patients with metastatic spine tumors. Patient enrollment began in August 2013 and ended in February 2017. Research ethics board approval was obtained at each center. Patients between 18 and 75 years old, treated for sacral metastases (S1 to S5) with surgery and/or radiotherapy were included. Patients were excluded if the primary site of cancer was the central nervous system or spine.
Demographic data, initial Spine Instability Neoplastic Score (SINS), information regarding the oncologic status and treatment data were collected at baseline.
For this study, patients included had at least 12 weeks follow up data completed with data collected at 6 weeks, 3 months and 6 months. A patient was lost to follow-up if he/she did not come for the scheduled study visit. Prior to declared lost to follow-up, three phone calls with at least 2 days in between each call were executed by the study personnel.
Outcomes measures
The baseline status of all patients was assessed using a variety of HRQOL outcome measures including the Spine Oncology Study Group Outcomes Questionnaire (SOSGOQv2.0), the Short Form-36 version 2 (SF-36v2) and the EuroQol-5Dimension (EQ-5D). SOSGOQv2.0 was developed specifically for the metastatic spine population (4). It encompasses 6 domains: physical function, neural function, pain, mental health, social function and post therapy questions. SOSGOQ scores were calculated according to the revised scoring system of the SOSGOQ2.0. A higher score corresponds with a higher level of functioning for the physical function and social function and a lower level of neurological symptoms, pain and symptoms for the mental health domain. The SF-36v2 and EQ-5D are generic measures of patient health status (5,6).
In addition, AEs were followed prospectively using a predefined list of common AEs. Neurologic examination with the ASIA (American Spinal Injury Association) motor score and bowel and bladder function were recorded at baseline and at follow up. Pain was assessed with the numeric rating scale (NRS) (7).
Statistical analysis
Standard descriptive statistics were used to represent demographic data. Differences in baseline parameters were tested by using Fisher’s exact test for categorical variables and t-test or Wilcoxon rank sum test for continuous variables. A mixed effect model was used to test for differences in patient reported outcome compared to baseline and between both treatments. P values were adjusted due to multiple testing by Tukey-Kramer. AE data was analyzed per patient and per AE time by using AEs which occurred up to 6 months after treatment. Confidence intervals for AE percentage were calculated using the exact binomial method.
All statistical analyses were performed using SAS (version 9.4, SAS Institute Inc., Cary, NC, USA). Significance was defined as P<0.05.
Results
Study population
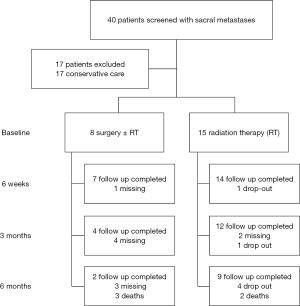
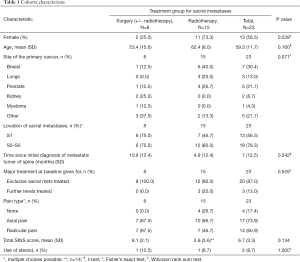
A total of 40 patients with sacral metastases were screened for inclusion, of which 23 satisfied study inclusion and exclusion criteria (Figure 1). Eight patients underwent surgery ± RT and 15 patients underwent RT alone. At 6 months follow up, 3 (37.5%) surgical patients and 2 (13.3%) RT patients were deceased. Table 1 gives a comparative breakdown of the demographic and tumor characteristics of the population.
Full table
Surgery ± RT
All patients underwent a single posterior procedure. Table 2 summarizes surgical details. Four patients had tumor in S1 extending lower in the sacrum (S2–S5), two had tumor confined to S1 only, and two patients had tumor located from S2–S5. Preoperative embolization was performed in three patients. One patient required a flap for closure. Two patients had postoperative adjuvant therapy and three had surgery after prior history of radiotherapy (more than 2 months before). Adjuvant RT was given at two months postoperatively (one received conventional RT and the other, SBRT). No patients died during the surgical admission.

Full table
RT
In the RT group, 7 (46.7%) received stereotactic body radiotherapy (SBRT) and 8 (53.3%) conventional radiotherapy. For SBRT, the mean dose was 27.4 (SD 3.2) Gy with a mean number of fraction of 3.3 (range: 2–5). For patients who underwent conventional RT, the mean dose was 17.5 (SD 10.9) Gy with a mean number of fraction of 5 (range: 1–12). No heavy particle radiation was used.
HRQOL
SOSGOQv2.0
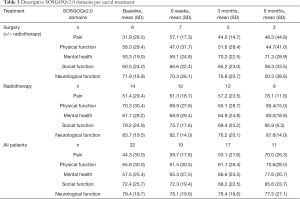
The postoperative SOSGOQv2.0 scores were better than the preoperative score at any time points for the surgical cohort. The baseline score for the RT group was higher than the surgical cohort and improved at 6 weeks and 6 months. The results of each domain at every follow up time are presented in Table 3.
Full table
SF-36v2
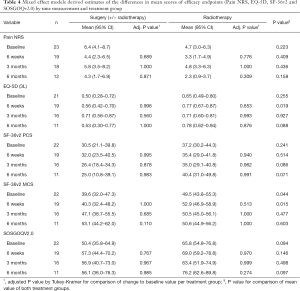
As expected, the baseline values for the SF-36v2 PCS for both cohorts were lower than the general population. The PCS value for the surgical cohort improved slightly initially and then decreased at longer follow-up. Conversely, the RT group scores decreased at early follow-up and improved thereafter (Table 4). The differences from baseline at different time points and between the 2 cohorts did not reach statistical significance either for the SF-36v2 PCS.
Full table
Worse SF-36v2 MCS score were observed at baseline for the surgical group (P=0.044). Both groups achieved values close to the normative population at 3 and 6 months with higher improvement in the surgical group (Table 4).
EQ-5D
For the entire cohort, the observed mean pre-treatment EQ-5D score was 0.60 (SD 0.28). It improved post treatment gradually to 0.73 (SD 0.24) at 6 months. The surgical cohort started with worse preoperative scores compared to the RT group (P=0.255) (Table 4).
Pain
Pain at baseline was different between groups. Pain in the surgical cohort was experienced by 7 of the 8 patients and clearly showed a predominance of mechanical pain: 57.1% of the axial pain was mechanical alone, 28.6% was both mechanical and biological. In the RT group, in the 10/15 patients who experienced pain, axial and radicular pain was predominantly biological (70% and 71.4%).
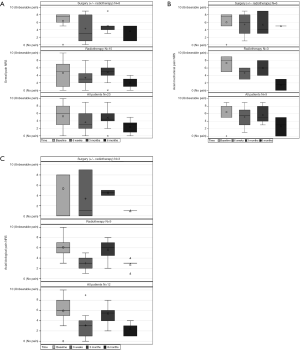
The overall NRS pain improved from the pre-treatment level in the whole population. A worse pain score was observed at baseline in the surgical group but did not reach statistical significance. Both groups improved at 6 weeks and at 6 months although this was not statistically significant (P>0.05) (Table 4). In the surgical group, six patients experienced axial mechanical pain pre-operatively with a median NRS pain score of 7.5 (IQR: 5–8). The median pain improved progressively at 6 weeks, 3 and 6 months. In the RT group, 3 patients had mechanical axial pain which showed improvement over time (Figure 2).
Neurologic function
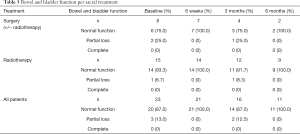
None of the 23 patients had complete loss of bowel and bladder function. Pre-treatment, 2 patients in the surgical group and 1 patient in the RT group experienced partial bowel and bladder loss. Two of these patients recovered normal function at 6 weeks and 1 patient in the surgical group was lost to follow up (Table 5). Using the SOSGOQv2.0 specific question regarding bladder and bowel function, the impact on HRQOL of the deficits appeared to be mild and stable in both cohorts.
Full table
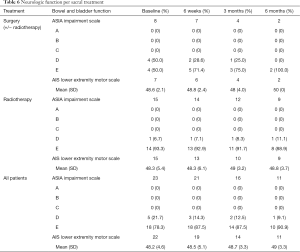
At baseline, the mean lower extremity motor score was 48.4 (range: 29–50) for the overall cohort and was similar at 3-month follow-up (48.7). Interestingly, neurologic deficit tended to be stable over time in the RT cohort. In the surgical cohort, one patient showed neurologic improvement at 6 weeks and the other patient was lost to follow up (Table 6). At baseline, the neurologic function domain of the SOSGOQv2.0 was worse in the surgical group compared to the RT group: mean 71.9 (18.9) vs. 83.7 (19.5). The scores in both groups tended to remain nearly stable over time. The lower extremity motor function domain of the SOSGOG however remained stable at 12 weeks in the RT group and slightly improved in the surgical cohort.
Full table
AEs
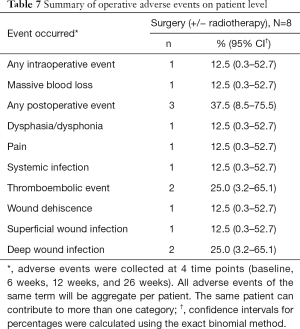
A total of 10 AEs occurred in 3 patients of the surgical cohort. Wound infection (n=3) was the most common postoperative AEs. Two patients experienced thromboembolic events and 1 patient presented with a systemic infection. Only one intra-operative AE was reported (massive blood loss) (Table 7).
Full table
AEs related to chemotherapy and/or RT occurred 10 times in four (66.7%) patients in the surgical cohort and 24 times in 7 (46.7%) patients in the RT cohort (Table 8). An L5–S1 neuritis was observed in one patient following surgery combined with RT. Pain flare was reported in 2 cases following RT.
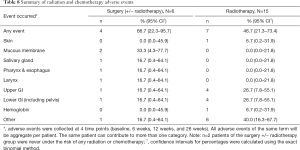
Full table
Discussion
This is the first study to assess HRQOL outcomes, a key outcome measure, in patients with symptomatic sacral metastases. Moreover, this paper represents the largest retrospective study of a prospective cohort of sacral metastases treated with either surgery and/or RT. This analysis demonstrated that these patients improved their pain and their quality of life with treatment. Using a generic (EQ-5D) tool, HRQOL increased maximally at 3 months in the surgical cohort and then declined, likely secondary to progression of systemic disease. On the other hand, early and sustained modest improvement in HRQOL was found when using a disease specific tool (SOSGOQv2.0). Improvement in HRQOL was less dramatic in the radiation cohort but is likely due to a ceiling effect as their baseline HRQOL was substantially better.
Interestingly, looking at surgery in other metastatic spinal locations, the improvement in the EQ-5D compares favourably (8). de Ruiter et al. reported an improvement of 0.15 at 3 months following surgical intervention for tumors predominantly located in the thoracic and lumbar spine, compared to a 0.22 improvement at 3 months in our study (9). Although the minimum clinically important difference (MCID) thresholds for EQ-5D has not been determined in the metastatic spine population, and a definition of MCID thresholds is inconsistent throughout the literature, it has been fixed at 0.1 by Wilson in patients with chronic pain (10). As pain palliation is the main surgical indication in this population, we believe that the improvement in EQ-5D is clinically significant although our results should be interpreted with caution due to low numbers.
The main objective of this study was not to compare different treatment modalities for sacral metastases, but to describe this population and provide a better understanding of treatment outcomes. Nevertheless, interesting findings emerged. Surgical patients consistently had worse baseline pain and HRQOL. The nature of the pain was also different: surgical patients had a predominance of mechanical pain resulting from instability whereas radiation patients suffered from biological pain resulting from tumor invasion. Mechanical pain is related to instability and typically is exacerbated with movement and relieved with recumbency. Biological or tumoral pain is a pain that is constant and not modified by movement. Pain improved significantly in both groups. This could be explained by the instantaneous stability conferred by surgical stabilisation, which was the driving surgical indication observed in our cohort. Although it would be expected that patients with mechanical pain improved with surgery, it was interesting that patients with mechanical pain who underwent RT alone also showed improvement. This limits the generalization that mechanical pain equals surgery, but the samples size is too small to make any conclusions. As neurological compression in sacral metastases may be secondary to tumors extending into the foramen and ventrally to the sacrum, surgery may not provide an appropriate decompression and may thus be futile to restore neurologic function. Nonetheless, surgical decompression remains a valid treatment when progressive neurological deficits are seen secondary to focal spinal canal involvement.
A systematic review on the management of metastatic sacral tumors published in 2012 revealed two prospective case-series that addressed these tumors: Gerszten et al. (11) reported on local control rate for 103 sacral tumors treated with radiosurgery and Akasu et al. (3) described survival and local control for abdominal sacral resection for rectal cancer (12). The other studies included were retrospective or case reports (13-23). More recently, Feiz-Erfan et al. (24) retrospectively reported that patients with sacral metastases showed significant and sustained pain improvement following surgery. Similarly to Feiz-Erfan et al. (24), we showed that surgical treatment is associated with marginal improvement in motor score and preservation of sphincter function. Du et al. (25) were the first to report HRQOL for this population using the QLQ-C30, a cancer specific outcome tool. In their retrospective study of 154 patients, QLQ-C30 improvement at 3 months post-operatively was observed. However, no information regarding loss to follow up and missing data were reported, limiting generalizability. Finally, our surgical related AE rate of 37.5% compares favourably to other contemporary case-series on sacral metastases (24,25).
This study adds to the literature by showing that select patients should be considered for surgery, especially when there is a stability issue or localized neurologic compromise in a radio-resistant tumor. Many tools are available to overcome the inherent difficulties associated with these cases. Multidisciplinary management is paramount. Pre-operative embolization is useful in avoiding transfusions and significant blood loss (26,27). The cell saver is a promising adjunct. Recent evidence supports the use of cell saver in surgery for spinal metastases. The absence of viable tumor cells in the salvaged blood has been demonstrated (28-31). Kumar et al. have shown that tumor cells that passed through the cell saver device, with or without the leucocyte depletion filter, are morphologically altered and have lost the ability to form new metastatic deposits (32). A recent systematic review on the safety and efficacy of lysine analogues in cancer patients did not show an increased risk of venous thromboembolism while being effective in reducing blood loss (33). Percutaneous sacroplasty is increasingly popular with a low rate of complication (34). Alternatively, neuropathic and neoplastic pain can effectively be controlled with neuromodulation options. Finally, good local control can be achieved with SBRT (35). This was observed in our study with only one patient showing progression at 6 months.
Limitations of this study are derived from the type of population studied and the relative rarity of sacral metastases. To overcome the rarity, this study was designed to be multicenter. Still, due to the small sample size, the ability to perform a detailed multivariate analysis was impossible. Furthermore, due to the nature of the population studied, follow up data were difficult to capture, even though a systematic approach was used. Finally, this study was performed in experienced centers, limiting generalization. However, this study is a representative sample of an underreported population. It may stimulate interest within the spinal community and lead to larger studies in the future.
Conclusions
Modern management of sacral metastases encompasses surgery and/or RT. Both alternatives appear to be reasonable therapeutic options. Based on patient symptomatology, more aggressive treatment, including surgery, may be beneficial. This cohort study described improvements in HRQOL and pain following both treatments. Furthermore, an acceptable AE rate and stabilisation of the neurologic deficits can be anticipated with either surgery and/or RT.
Acknowledgments
We are grateful to the collaborating centers local clinical research personnel and support staff for their active participation. This study was organized and funded by AOSpine International, through the AOSpine Knowledge Forum Tumor, a pathology-focused working group of up to ten international spine experts acting on behalf of AOSpine in the domain of scientific expertise. A research grant for this study was received from the Orthopedic Research and Education Foundation (OREF). Study support was provided directly through AOSpine’s Research department and AO’s Clinical Investigation and Documentation unit. This work was supported by the AOSpine Knowledge Forum Tumor (AOSKFT) and by an Orthopedic Research and Education Foundation grant.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Research ethics board approval was obtained at each center (No. NCT01825161) and written informed consent was obtained from all patients.
References
- Jacobs WB, Perrin RG. Evaluation and treatment of spinal metastases: an overview. Neurosurg Focus 2001;11:e10. [Crossref] [PubMed]
- Sundaresan N, Boriani S, Okuno S. State of the art management in spine oncology: a worldwide perspective on its evolution, current state, and future. Spine 2009;34:S7-20. [Crossref] [PubMed]
- Akasu T, Yamaguchi T, Fujimoto Y, et al. Abdominal sacral resection for posterior pelvic recurrence of rectal carcinoma: analyses of prognostic factors and recurrence patterns. Ann Surg Oncol 2007;14:74-83. [Crossref] [PubMed]
- Street J, Lenehan B, Berven S, et al. Introducing a new health-related quality of life outcome tool for metastatic disease of the spine: content validation using the International Classification of Functioning, Disability, and Health; on behalf of the Spine Oncology Study Group. Spine 2010;35:1377-86. [Crossref] [PubMed]
- McHorney CA, Ware JE, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993;31:247-63. [Crossref] [PubMed]
- EuroQol - a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. [Crossref] [PubMed]
- Hartrick CT, Kovan JP, Shapiro S. The Numeric Rating Scale for Clinical Pain Measurement: A Ratio Measure? Pain Practice 2003;3:310-6. [Crossref] [PubMed]
- Fehlings MG, Nater A, Tetreault L, et al. Survival and Clinical Outcomes in Surgically Treated Patients With Metastatic Epidural Spinal Cord Compression: Results of the Prospective Multicenter AOSpine Study. J Clin Oncol 2016;34:268-76. [Crossref] [PubMed]
- de Ruiter GCW, Nogarede CO, Wolfs JFC, et al. Quality of life after different surgical procedures for the treatment of spinal metastases: results of a single-center prospective case series. Neurosurg Focus 2017;42:E17. [Crossref] [PubMed]
- Wilson HD. Minimum clinical important differences of Health Outcomes in a chronic pain population; are they predictive of poor outcomes? University of Texas Arlington 2008:1-230.
- Gerszten PC, Burton SA, Ozhasoglu C, et al. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine 2007;32:193-9. [Crossref] [PubMed]
- Quraishi NA, Giannoulis KE, Edwards KL. Management of metastatic sacral tumours. Eur Spine J 2012;21:1984-93. [Crossref] [PubMed]
- Albareda J, Herrera M, Lopez Salva A, et al. Sacral metastasis in a patient with endometrial cancer: case report and review of the literature. Gynecol Oncol 2008;111:583-8. [Crossref] [PubMed]
- Fujibayashi S, Neo M, Nakamura T. Palliative dual iliac screw fixation for lumbosacral metastasis. Technical note. J Neurosurg Spine 2007;7:99-102. [Crossref] [PubMed]
- Kakutani K, Doita M, Nishida K, et al. Radiculopathy due to malignant melanoma in the sacrum with unknown primary site. Eur Spine J 2008;17 Suppl 2:S271-4. [Crossref] [PubMed]
- Kollender Y, Meller I, Bickels J, et al. Role of adjuvant cryosurgery in intralesional treatment of sacral tumors. Cancer 2003;97:2830-8. [Crossref] [PubMed]
- Menegaz RA, Resende AD, da Silva CS, et al. Metastasis of choriocarcinoma to lumbar and sacral column. Eur J Obstet Gynecol Reprod Biol 2004;113:110-3. [Crossref] [PubMed]
- Nebreda C, Vallejo R, Aliaga L, et al. Percutaneous sacroplasty and sacroiliac joint cementation under fluoroscopic guidance for lower back pain related to sacral metastatic tumors with sacroiliac joint invasion. Pain Pract 2011;11:564-9. [Crossref] [PubMed]
- Ozdemir MH, Gürkan I, Yildiz Y, et al. Surgical treatment of malignant tumours of the sacrum. Eur J Surg Oncol 1999;25:44-9. [Crossref] [PubMed]
- Toro A, Pulvirenti E, Manfrè L, et al. Sacroplasty in a patient with bone metastases from hepatocellular carcinoma. A case report. Tumori 2010;96:172-4. [Crossref] [PubMed]
- Turgut M, Gökpinar D, Barutça S, et al. Lumbosacral metastatic extradural Merkel cell carcinoma causing nerve root compression--case report. Neurol Med Chir (Tokyo) 2002;42:78-80. [Crossref] [PubMed]
- Uemura A, Matsusako M, Numaguchi Y, et al. Percutaneous sacroplasty for hemorrhagic metastases from hepatocellular carcinoma. AJNR Am J Neuroradiol 2005;26:493-5. [PubMed]
- Zhang J, Wu CG, Gu YF, et al. Percutaneous sacroplasty for sacral metastatic tumors under fluoroscopic guidance only. Korean J Radiol 2008;9:572-6. [Crossref] [PubMed]
- Feiz-Erfan I, Fox BD, Nader R, et al. Surgical treatment of sacral metastases: indications and results. J Neurosurg Spine 2012;17:285-91. [Crossref] [PubMed]
- Du Z, Guo W, Yang R, et al. What Is the Value of Surgical Intervention for Sacral Metastases? Tsuchiya H, editor. PLoS One 2016;11:e0168313.
- Ashour R, Aziz-Sultan A. Preoperative tumor embolization. Neurosurg Clin N Am 2014;25:607-17. [Crossref] [PubMed]
- Pikis S, Itshayek E, Barzilay Y, et al. Preoperative embolization of hypervascular spinal tumors: current practice and center experience. Neurol Res 2014;36:502-9. [Crossref] [PubMed]
- Kumar N, Ahmed Q, Lee VKM, et al. Are we ready for the use of intraoperative salvaged blood in metastatic spine tumour surgery? Eur Spine J 2016;25:3997-4007. [Crossref] [PubMed]
- Kumar N, Zaw AS, Ahmed Q, et al. Are we ready for transfusing intraoperative salvaged blood in metastatic spine tumour surgery. Spine J 2016;16:S61.
- Kumar N, Ravikumar N, Tan JYH, et al. Current Status of the Use of Salvaged Blood in Metastatic Spine Tumour Surgery. Neurospine 2018;15:206-15. [Crossref] [PubMed]
- Elmalky M, Yasin N, Rodrigues-Pinto R, et al. The safety, efficacy, and cost-effectiveness of intraoperative cell salvage in metastatic spine tumor surgery. Spine J 2017;17:977-82. [Crossref] [PubMed]
- Kumar N, Zaw AS, Khoo BL, et al. Intraoperative cell salvage in metastatic spine tumour surgery reduces potential for reinfusion of viable cancer cells. Eur Spine J 2016;25:4008-15. [Crossref] [PubMed]
- Montroy J, Fergusson NA, Hutton B, et al. The Safety and Efficacy of Lysine Analogues in Cancer Patients: A Systematic Review and Meta-Analysis. Transfus Med Rev 2017;31:141-8. [Crossref] [PubMed]
- Kortman K, Ortiz O, Miller T, et al. Multicenter study to assess the efficacy and safety of sacroplasty in patients with osteoporotic sacral insufficiency fractures or pathologic sacral lesions. J Neurointerv Surg 2013;5:461-6. [Crossref] [PubMed]
- Gerszten PC, Mendel E, Yamada Y. Radiotherapy and radiosurgery for metastatic spine disease: what are the options, indications, and outcomes? Spine 2009;34:S78-92. [Crossref] [PubMed]