
Modification of the tumor response threshold in patients of advanced non-small cell lung cancer treated with chemotherapy plus targeted agents: a pooled study from five clinical trials in one institution
Introduction
Lung cancer is by far the leading cause of cancer death worldwide, of which around 80–85% patients are classified as non-small cell lung cancer (NSCLC) (1-4). Approximately 30–40% of NSCLC patients are diagnosed locally advanced or metastatic at their initial diagnosis. Systemic chemotherapy is now recommended to be the main therapeutic method for these patient population (5). However, the standard chemotherapy regimens of platinum-based double-agent has come to its therapeutic platform, with an overall survival (OS) of 8 months, progression-free survival (PFS) of 3.7 months and 1-year survival rate at 33% (6). Chemotherapy has traditionally played such a role in the treatment of advanced NSCLC (7), however, as the booming development of molecular targeted drugs in recent years, various novel small molecule inhibitors and monoclonal antibody came out with astonishing therapeutic efficacy and low toxicity. For instance, the IPASS study in 2009 was the first time a targeted monotherapy has demonstrated significantly longer PFS than doublet chemotherapy (8). Followed by multiple clinical trial design on various targeted new drug into the exploration of new therapeutic pattern. The combined strategies were described as a promising therapeutic pattern of NSCLC, which was hopeful in improving survival benefit (9). The combinational therapy of chemotherapeutic agents with targeted agents, including angiogenesis inhibitors, epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) and tumor cell apoptosis inducers etc., has been studied as a new therapy option for NSCLC. ECOG4599, a phase III randomized controlled study has confirmed the efficacy and safety of bevacizumab plus paclitaxel and carboplatin in patients with recurrent or advanced NSCLC, with 2 months superior to the control group in OS (10). Followed by Zhou in BEYOND study revealed that bevacizumab cooperated well with carboplatin and paclitaxel in Chinese patients with advanced non-squamous NSCLC and led to a clinically meaningful treatment benefit (11). Besides, INTACT–II study confirmed the safety of Gefitinib plus paclitaxel and carboplatin in chemotherapy-naive patients with advanced NSCLC (12). Improvement in PFS was uniformly observed in a phase II clinical trial where the combination of chemotherapy plus erlotinib was treated for advanced NSCLC patients as first-line treatment, which was further confirmed by the FASTACT-II study (13,14). In addition, preliminary results of the latest phase III study, designed to compare the efficacy and tolerability of single gefitinib with the combination of gefitinib and chemotherapy (pemetrexed plus carboplatin) in advanced NSCLC patients harboring EGFR mutation, demonstrated the superiority of the combined treatment with regard to survival prognosis (15). This novel combinational therapeutic pattern has offered a brand-new option for the management of advanced NSCLC. Response Evaluation Criteria in Solid Tumors (RECIST) has been extensively applied in not only treatment decisions in individual patient care but also in clinical trials response evaluation to assess the efficacy of new drugs during the process of drug development and regulatory approvals. The treatment effect was usually stratified by RECIST into complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD), where PR was defined as at least a 30% decrease in the sum of longest diameters of target lesions (16).
The RECIST criteria provide standardized, objective measurements of solid tumor response to anti-cancer therapy, however, it’s known that the RECIST criteria were generated based on masses of data from cytotoxic chemotherapy clinical trials and its limitation for assessing therapy with molecularly targeted agents have been increasingly recognized. A follow-up validation study in metastatic renal cell carcinoma also showed that 10% tumor shrinkage is a reliable early predictor of survival outcome for patients receiving VEGFR-targeted therapies (17). And a recent meta-analysis also showed that colorectal cancer patients with 20% reduction in the sum of the longest diameter (SLD) of target lesions were likely to have a better OS and PFS when compared with non-responders patients who were less than 20% reduction in the SLD (18). In clinical practice, we found that those NSCLC patients treated with targeted agents who achieved a less than 30% tumor shrinkage also seem to be in good response and finally predicted survival benefit from the treatment, which was not always corresponding with the RECIST criteria (19-21). Therefore, we assume there is an optimal threshold for this combinational therapeutic method setting which may be more clinically meaningful than standard RECIST criteria. And by applying to X-tile analysis, we seek to detect a more appropriate shrinkage threshold of therapy response with the data available from five clinical trials in one institution, which can better distinguish responders from non-responders in NSCLC patients receiving combinational chemotherapy plus targeted therapy.
Methods
Patients
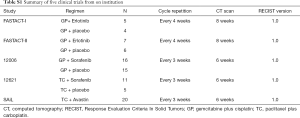
Patients with stage IIIB or IV NSCLC were enrolled in 5 clinical trials (FASTACT-I, FASTACT-II, 12621, 12006 and SAiL study) from Sun Yat-sen University Cancer Center (13,14,22-24). Patients who received targeted therapy (Erlotinib, Sorafenib and Bevacizumab) combining with chemotherapy as first-line treatment in Sun Yat-sen University Cancer Center from September, 2006 to August, 2011, were retrospectively analyzed. The criteria for eligibility were listed as follows: (I) histologically or cytologically-confirmed stage IIIB or IV NSCLC; (II) an age of at least 18 years; (III) an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; (VI) having at least one measurable target lesion; (V) possessing adequate hematologic, hepatic, and renal function; (VI) having a life expectancy of at least 3 months. Patients who had received systemic treatment, including target therapy, chemotherapy or radiotherapy within 4 weeks of study were excluded; patients with brain metastases were ineligible for the studies. All patients signed informed consent forms. These studies were all approved by the Ethics Committee of Sun Yat-sen University Cancer Center (YB2015-080). The process was conducted in accordance with the Declaration of Helsinki and good clinical practice.
Of the 96 patients enrolled, 4 patients in the 12006 study and 2 patients in the SAiL study even had not completed a cycle treatment, and 1 patient dropped out after 1 cycle treatment due to AE in the 12621 study. A total of 39 patients from the control group (chemotherapy alone or chemotherapy with placebo) were all excluded in this study since we focus on the patient population treated with combinational chemotherapy plus targeted therapy. A sum of 59 patients was eventually evaluated, with 25 females and 34 males. The median age was 53 years (range, 25–78 years). The clinical characteristics of the enrolled patients in these 5 clinical trials were summarized in Table S1. Clinical outcome data were collected by review of medical records and case report forms, including date of progression (progression as determined by RECIST) and date of death.
Full table
Treatment regimens
In the 12621 study, patients received 175 mg/m2 of paclitaxel/carboplatin (TC) at a dose calculated to produce an AUC (area under the concentration–time curve) of 5 mg/mL·min on day 1, and Sorafenib/placebo 400 mg twice a day on day 1–21. The cycle was repeated every 3 weeks.
In the 12006 study, patients treated with gemcitabine, at a dose of 1,250 mg/m2 administered on day 1 and 8, 75 mg/m2 of cisplatin administered on day 1 (GP), and Sorafenib/placebo 400 mg twice per day, for a cycle of 3 weeks. In both studies patients continued to receive Sorafenib or placebo until progression or unacceptable toxicity or death after 3 cycles of treatment.
In the FASTACT-I/II study, patients were administered 6 cycles of gemcitabine at a dose of 1,250 mg/m2 on days 1 and 8, followed by cisplatin at a dose of 75 mg/m2 or carboplatin 5× AUC on day 1 (GP), and with intercalated Erlotinib/placebo 150 mg/day on day 15–28 every 4 weeks. In the same way, patients continued to receive Erlotinib or placebo until progression or unacceptable toxicity or death, and all patients in the placebo group were offered second-line Erlotinib at the time of progression.
In SAiL study, patients were all treated with paclitaxel at a dose of 175 mg/m2 and carboplatin at a dose calculated to produce an AUC of 5 mg/mL·min on day 1 (TC), together with Bevacizumab 15 mg/kg every 3 weeks in this open-label, single-arm phase IV study.
We analyzed all the 59 evaluable patients in these 5 trials. Among the 16 patients from 12621 study, 11 received GP plus Sorafenib, while others received GP plus placebo. Of the 31 patients from 12006 study, 16 were treated with GP plus Sorafenib, with the remaining received GP plus placebo. Among the 9 patients from FASTACT-I study, 5 received GP/GC plus Erlotinib, and 5 received GP/GC plus placebo. And the 13 patients from FASTACT-II study: 7 of them received GP/GC plus Erlotinib, and the other 6 received GP/GC plus placebo. The rest of 20 patients were from SAiL study, which were all treated with TC plus Bevacizumab.
Follow-up
The 59 patients received CT scan every 6 or 8 weeks until disease progression or death according to the protocols of these 5 clinical trials. The detailed information of these five clinical trials was listed in Table S1 as follows.
Tumor response evaluation
The target lesion was assessed with computed tomography (CT) or magnetic resonance imaging (MRI) at baseline before the treatment, RECIST 1.0 evaluation of therapeutic effect was based on tumor assessment with follow-up, including chest and upper-abdominal CT scan every 2 cycles of treatment or every 6 weeks until disease progression or death. Besides, the method of assessment was exactly the same as that in the corresponding clinical trials, and the imaging evaluation was performed by an independent radiologic review committee. Patients were classified into PR and PD, was recorded at CT follow-up, based on ≥30% decrease in SLD for PR and ≥20% increase in SLD for PD, SD was defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as reference the smallest sum diameters while on study, according to RECIST 1.0. In this current study, whereas RECIST response was studied according to RECIST both 1.0 and 1.1, the RECIST categorizations are thought to be comparable, since the definitions of PR, PD, and SD remain unchanged.
Selection of cutoff value
X-tile program was applied to determine the optimal value of tumor shrinkage based on PFS and OS outcome according to Camp et al. (25).
Analysis of alternate response thresholds
For each tested threshold of −10%, chemotherapy plus targeted agents-treated patients with ∆SLDs larger than this threshold was defined as responders and those with ∆SLDs smaller than this threshold was defined as potential non-responders. The median PFS and OS for each subgroup was estimated using Kaplan-Meier estimates and the ratio of median PFS and OS for responders versus potential non-responders was calculated. If the 95% confidence interval (CI) of the ratio of median PFS and OS did not include 1, the PFS and OS of the two subgroups was considered to be different.
Statistics analysis
We compared the difference of patients’ survival by means of PFS and OS. PFS was calculated from the date of randomization to disease progression (local or metastatic) or death for any reason. OS was defined as the time elapsed from the date of randomization to the time of death for any cause. Kaplan-Meier method was used to describe PFS and OS. The survival distribution difference in the two groups was evaluated using the log-rank test. 95% CI was calculated for PFS and OS outcomes to assess the treatment efficacy. Univariate analyses and multivariate analyses were performed using Cox proportional hazard models in an exploratory fashion to explore any effect between the decrease in SLD and survival outcomes. A P value of less than 0.05 was considered statistically significant. Statistical Package for Social Sciences (SPSS) 23.0 software was used for all statistical analysis (IBM, Armonk, NY, USA).
Results
Patients
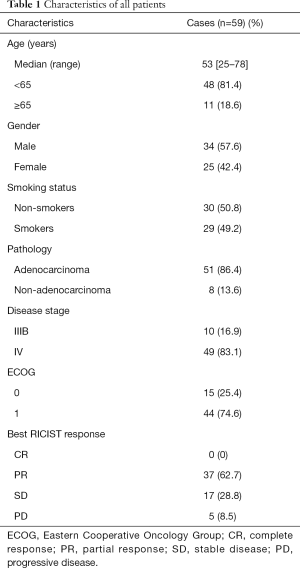
This retrospective analysis included 59 patients. The median follow-up time was 24.739 months. Baseline characteristics of all the patients were reported in Table 1. The median age was 53 years (range, 25–78 years). Of these 59 patients, 25 patients (42.4%) were female. Among these enrolled patients, 30 (50.8%) were non-smokers. All 59 patients were evaluated by RECIST and 10.0% tumor shrinkage thresholds respectively. According to RECIST criteria, the objective response rate (ORR) (CR + PR) was 62.7% (Table 1). According to 10.0% tumor shrinkage thresholds, 50 (84.7%) patients were considered responders whom achieved more than 10.0% tumor shrinkage, whereas 9 (15.3%) patients achieved less than 10.0% tumor shrinkage were deemed as non-responders. Patients’ characteristics according to the new cutoff analyzed subgroups are shown in Table S2 and the waterfall plot (Figure 1) showed the changes in the sum of long axis diameter (SLD) of target lesions in percentage from baseline to best overall response’ follow-up evaluation.
Full table
Full table

Threshold evaluation by X-tile program
X-tile program was applied to demonstrated the optimal cut-off value for tumor shrinkage thresholds based on PFS was −10% (χ2=15.8031, P=0.0026) and the optimal cut-off value for tumor shrinkage thresholds based on OS was also −10% (χ2=11.3351, P=0.0207) (Figure 2). The whole patients were divided into two groups in accord with the cutoff value: responders, the SLD of target lesions decrease by more than 10% and non-responders, the SLD of target lesions decrease by less than 10%.
Kaplan-Meier estimates in PFS and OS according to RECIST criteria and −10% in ∆SLD threshold
The median PFS time was 8.805 (95% CI, 5.785 to 11.825) months. With new-cutoff of 10.0%, Kaplan-Meier survival analysis and log-rank test revealed that 10.0% tumor shrinkage in SLD can distinctly discriminated responder patients from non-responder patients (responders vs. non-responders, median PFS, 10.1 vs. 2.50 months, P=0.0007, Figure 3A). When referring to RECIST, the PFS for CR + PR patients was statistically significant when compared with SD + PD patients (CR + PR vs. SD + PD, median PFS, 10.55 vs. 6.18 months, P=0.064, Figure 3B).
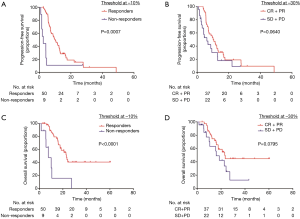
The median OS time was 22.669 (95% CI, 15.885 to 29.453) months. According to Kaplan-Meier survival analysis and log-rank test, notably the results demonstrated 10.0% decrease in SLD significantly predictive of OS (responders vs. non-responders, median OS, 23.00 vs. 7.66 months, P<0.0001, Figure 3C). However, when referring to RECIST, the OS for CR + PR patients were not significantly different from SD + PD patients (CR + PR vs. SD + PD, median OS, 23.00 vs. 15.97 months, P=0.0795, Figure 3D).
Univariate and multivariable cox regression analyses
The results of the univariate and multivariable analysis of the correlation between clinical characteristics and prognosis of the 59 patients are listed as follows.
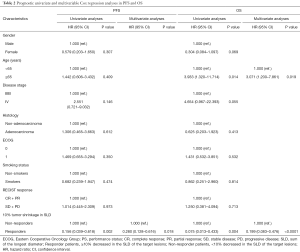
For PFS, an obvious statistically significance was also observed in 10.0% tumor shrinkage in SLD for non-responders versus responders [HR 1.000 (ref.) vs. 0.156; 95% CI, 0.039–0.618, P=0.002] in univariate analysis. In multivariate analysis, the results further confirmed 10.0% tumor shrinkage in SLD a valid prognostic factors for PFS [HR 1.000 (ref.) vs. 0.280; 95% CI, 0.128–0.616, P=0.018] (Table 2).
Full table
For OS, according to univariate analysis, age (P=0.014) and 10.0% tumor shrinkage in SLD (P=0.004) were independent prognostic factors. Multivariate analyses also proved age [HR 1.00 (ref.) vs. 3.071; 95% CI, 1.200–7.861, P=0.019] and 10.0% tumor shrinkage in SLD for non-responders versus responders [HR 1.00 (ref.) vs. 0.199; 95% CI, 0.083–0.476, P<0.0001] as valid prognostic factors (Table 2).
Discussion
We retrospectively analyzed the data of advanced NSCLC patients receiving combinational chemotherapy with targeted agents from five clinical trials and reevaluated the correlation between therapeutic efficacy and survival benefit with the RECIST response criteria and newly identified cutoff value in ∆SLD. We use X-tile analysis and Kaplan-Meier method to explore the optimal threshold change in SLD in the whole follow-up of clinical trial for tumor response assessment. In our study of 59 patients, we found that a 10% decrease in SLD in target lesion was predictive of survival in PFS and OS outcomes and might be a new cutoff for therapeutic response evaluation based on the data available from the former clinical trials.
We first used X-tile method to analyze optimal threshold for response or non-response group in our study, similar to Camp et al. (25). As a result, we finally identified the −10% threshold with the maximal sensitivity and specificity, which seemed to best differentiate patients with prolonged survival from those who would not. In our current study, a number of 59 eligible patients were finally enrolled and analyzed, with 25 females and 34 males. The median OS was 22.669 months (95% CI, 15.885 to 29.453) and median PFS was 8.805 months (95% CI, 5.785 to 11.825) for all the patient population. According to the RECIST criteria, the ORR (CR + PR) was 62.7%, compared with the 84.7% were deemed as responder patients when using −10% in ∆SLD threshold.
The correlation between tumor shrinkage and clinical outcome in PFS and OS was analyzed respectively. The result shows that in both PFS and OS outcome, with the −10% in ∆SLD threshold that we tested, the population were separated into two distinct subgroups in PFS (responders vs. non-responders, median PFS, 10.1 vs. 2.50 months, P=0.0007) and OS (responders vs. non-responders, median PFS, 23.00 vs. 7.66 months, P<0.0001) when using the Kaplan-Meier method. Oppositely, RECIST criteria could not separate the CR + PR group from SD + PD group in PFS (CR + PR vs. SD + PD, median PFS, 10.55 vs. 6.18 months, P=0.064) and OS (CR + PR vs. SD + PD, median OS, 23.00 vs. 15.97 months, P=0.0795). Furthermore, univariate and multivariable Cox regression analyses were taken to determine the correlation between survival outcome (PFS and OS) and clinical characteristics, revealing that 10.0% tumor shrinkage in SLD (P=0.018) was valid prognostic factors for PFS (Table 2) while age (P=0.019) and 10.0% tumor shrinkage in SLD (P<0.0001) were also found valid prognostic factors in OS (Table 2).
However, some limitations should be considered in this study. First, it is a retrospective study focusing on NSCLC patients receiving chemotherapy plus targeted agents; patients were pooled from five multicenter clinical trials from only one institution, which resulted in a small sample size of our study. Moreover, there are a limited number of the clinical trials on combinational chemotherapy with targeted therapy available for us to enroll more patients into this study. In addition, this study, on the basis of retrospective single center analysis, is lack of external validation. However, these patients were strictly enrolled according to inclusion and exclusion criteria and then received standard treatment with a long-term strict and regular follow-up visit. Besides, the imaging evaluations of these patients were carried out by a systematical radiologic review committee. Therefore, the results of the present study could still be informative. Second, there is an obstacle for treatment response assessment in solid tumors when using tumor thresholds. It is unavoidable, with any categorizing strategy including RECIST criteria, that two patients with close responses could be categorized into two separate groups (non-responders vs. responders) (26,27).
The RECIST criteria (16,28) are widely applied to evaluating the treatment response of solid tumors in various clinical trials and daily practice. A ’Partial Response’ is defined by a 30% reduction in the sum of the largest diameters (SLD) of the target lesions. However, this −30% threshold doesn’t seem to well adapt for the assessment of targeted therapies efficacy as well as the combinational therapies setting. It is known to us that the aim of target therapy is often to cause tumor necrosis and inhibit the growth of tumor, which is different from the killing-effect of cytotoxic drugs. Since RECIST criteria were summarized and established based on data from cytotoxic chemotherapy clinical trials, its limitation for assessing therapy with molecularly targeted agents have been increasingly emphasized. When considering this different therapeutic mechanism and influencing factors, it’s reasonable to deduce whether the traditional treatment effect evaluation RECIST criteria are still well adapted to this combinational therapeutic option in NSCLC patients. Early in 2010, Thiam has reported that 10% tumor shrinkage in SLD was predictive of PFS in a study of mRCC patients treated with sunitinib (29), which was further confirmed by a follow-up validation study (17). And a recent meta-analysis on advanced colorectal cancer also indicated that patients treated with chemotherapy plus target-agents with an early tumor shrinkage (defined as a variation of −10% to −20% in the SLD of target lesions) were associated with a better OS and PFS when comparing with patients who were deemed non-responders (<20% reduction in the SLD) (18). Our former study also revealed that the 8.32% tumor diameter shrinkage threshold was predictive of survival in advanced NSCLC patients treated with single target therapeutic agent (20).
To our knowledge, drug research and development is a lengthy, expensive, and complex process, of which clinical trial is the longest and the most expensive stage of drug development. Molecularly targeted agents, instead of chemotherapy killing-effect and leading to tumor necrosis, often stop tumor from further proliferation and remain disease stable. Lots of targeted-treated patients only reach disease control with tumor shrinkage in smaller magnitudes, may therefore have low ORRs as defined by RECIST. And notably, parts of these patients lived a comfortable end of life and achieved a final survival benefit eventually with the help of the new targeted agents. However, millions of new drugs came to an untimely end at very early stage, especially the phase I or II study during the clinical trial. These studies often take the RECIST-guided ORR as a primary end point and resulted in countless abortion in new drug research and development. For advanced solid tumor patients, the quality of life near the end their life is extremely important. These new targeted agents are worthy to be exploited and applied in clinical practice, for its strong binding affinity, high stability and relative low toxicity. It highline again that whether the existing RECIST criteria can reflect the true efficacy of a new targeted agent. Our current study, patients enrolled were homogeneous and all the clinical data were objective and of high quality, ensuring the truthful statistical analysis. Although we found that −10% in ∆SLD might be a better threshold only for this chemotherapy plus target agents setting, we sincerely hoped that our study could provide some new thoughts for tumor response assessments.
In our daily clinical practice, oncologists would have to take other parameters into account, such as clinical evaluation of symptoms and laboratory examination, when performing with thresholds to guide their therapeutic decisions. With the aid of the −10% in ∆SLD new cutoff, treatment efficacy can be demonstrated earlier and modified therapeutic decisions are likely to be carried out timely to stop futile treatment or avoid missing a promising new drug. As newer therapeutic agents become available in various clinical settings, it was required to further modify the existing tumor size-based response RECIST criteria and update the practical guidelines to meet the needs of drug development and patient care in this era of novel therapies. However, whether −10% or other cutoffs would be optimal for treatment decisions in individual patient care remained uncertain and some more sufficiently powered, prospective clinical trials were required for testifying and replenishing the current results.
Conclusions
A 10% tumor shrinkage in the SLD indicated a new threshold for identifying NSCLC patients who are benefiting from chemotherapy plus targeted therapy. This −10% threshold was further tested and confirmed to be a promising predictor of PFS and OS in this combinational therapeutic setting with the univariate and multivariate Cox regression analysis. Further prospective studies are required to further confirm these findings and to qualify its potential for the therapeutic effect evaluation in advanced NSCLC patients.
Acknowledgments
Funding: National Key R&D Program of China, Grant No. 2016YFC0905500, 2016YFC0905503. The 5010 Clinical Research Foundation of Sun Yat-sen University, Grant No. 2016001. The Natural Science Foundation of Guangdong Province of China, Grant No. 2018A0303130243. Science and Technology Program of Guangzhou, Grant No. 201607020031.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: These studies were all approved by the Ethics Committee of Sun Yat-sen University Cancer Center (YB2015-080).
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Chen W, Zheng R, Zeng H, et al. Epidemiology of lung cancer in China. Thorac Cancer 2015;6:209-15. [Crossref] [PubMed]
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- Novello S, Le Chevalier T. Chemotherapy for non-small-cell lung cancer. Part 2: Advanced disease. Oncology (Williston Park) 2003;17:457-64, 69-71; discussion 71, 78-80, 83-4.
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. Non-Small Cell Lung Cancer, Version 6.2015. J Natl Compr Canc Netw 2015;13:515-24. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Aggarwal C, Somaiah N, Simon G. Antiangiogenic agents in the management of non-small cell lung cancer: where do we stand now and where are we headed? Cancer Biol Ther 2012;13:247-63. [Crossref] [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. BEYOND: A Randomized, Double-Blind, Placebo-Controlled, Multicenter, Phase III Study of First-Line Carboplatin/Paclitaxel Plus Bevacizumab or Placebo in Chinese Patients With Advanced or Recurrent Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2197-204. [Crossref] [PubMed]
- Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 2. J Clin Oncol 2004;22:785-94. [Crossref] [PubMed]
- Wu YL, Lee JS, Thongprasert S, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol 2013;14:777-86. [Crossref] [PubMed]
- Mok TS, Wu YL, Yu CJ, et al. Randomized, placebo-controlled, phase II study of sequential erlotinib and chemotherapy as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 2009;27:5080-7. [Crossref] [PubMed]
- Inoue A, Hosomi Y, Maemondo M, et al. NEJ009 trial: A randomized phase III study of gefitinib (G) in combination with carboplatin (C) plus pemetrexed (P) versus G alone in patients with advanced nonsquamous non-small cell lung cancer (NSCLC) with EGFR mutation. J Clin Oncol 2014;32:TPS8131. -TPS. [Crossref]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Krajewski KM, Franchetti Y, Nishino M, et al. 10% Tumor diameter shrinkage on the first follow-up computed tomography predicts clinical outcome in patients with advanced renal cell carcinoma treated with angiogenesis inhibitors: a follow-up validation study. Oncologist 2014;19:507-14. [Crossref] [PubMed]
- Petrelli F, Pietrantonio F, Cremolini C, et al. Early tumour shrinkage as a prognostic factor and surrogate end-point in colorectal cancer: a systematic review and pooled-analysis. Eur J Cancer 2015;51:800-7. [Crossref] [PubMed]
- Zhou T, Zheng L, Hu Z, et al. The effectiveness of RECIST on survival in patients with NSCLC receiving chemotherapy with or without target agents as first-line treatment. Sci Rep 2015;5:7683. [Crossref] [PubMed]
- He X, Zhang Y, Ma Y, et al. Optimal tumor shrinkage predicts long-term outcome in advanced nonsmall cell lung cancer (NSCLC) treated with target therapy: Result from 3 clinical trials of advanced NSCLC by 1 institution. Medicine (Baltimore) 2016;95:e4176. [Crossref] [PubMed]
- Zhang J, Huang Y, Li X, et al. The impact of tumor size change after target therapy on survival: analysis of patients enrolled onto three clinical trials of advanced NSCLC from one institution. Onco Targets Ther 2012;5:349-55. [Crossref] [PubMed]
- Tsai CM, Au JS, Chang GC, et al. Safety and efficacy of first-line bevacizumab with chemotherapy in Asian patients with advanced nonsquamous NSCLC: results from the phase IV MO19390 (SAiL) study. J Thorac Oncol 2011;6:1092-7. [Crossref] [PubMed]
- Paz-Ares LG, Biesma B, Heigener D, et al. Phase III, randomized, double-blind, placebo-controlled trial of gemcitabine/cisplatin alone or with sorafenib for the first-line treatment of advanced, nonsquamous non-small-cell lung cancer. J Clin Oncol 2012;30:3084-92. [Crossref] [PubMed]
- Wakelee HA, Lee JW, Hanna NH, et al. A double-blind randomized discontinuation phase-II study of sorafenib (BAY 43-9006) in previously treated non-small-cell lung cancer patients: eastern cooperative oncology group study E2501. J Thorac Oncol 2012;7:1574-82. [Crossref] [PubMed]
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004;10:7252-9. [Crossref] [PubMed]
- Aras M, Erdil TY, Dane F, et al. Comparison of WHO, RECIST 1.1, EORTC, and PERCIST criteria in the evaluation of treatment response in malignant solid tumors. Nucl Med Commun 2016;37:9-15. [PubMed]
- Oxnard GR, Morris MJ, Hodi FS, et al. When progressive disease does not mean treatment failure: reconsidering the criteria for progression. J Natl Cancer Inst 2012;104:1534-41. [Crossref] [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [Crossref] [PubMed]
- Thiam R, Fournier LS, Trinquart L, et al. Optimizing the size variation threshold for the CT evaluation of response in metastatic renal cell carcinoma treated with sunitinib. Ann Oncol 2010;21:936-41. [Crossref] [PubMed]


