
Stereotactic body radiation therapy could improve disease control in oligometastatic patients with renal cell carcinoma: do we need more evidence?
Renal cell carcinoma (RCC) represents the most common type of kidney tumor and about 70% of the affected patients have clear cell histology tumors (1). Approximately 30% of the patients with RCC will recur locally or distantly after primary treatment and another 30% will present stage IV at diagnosis (2). Usually, distant disease involves lung and liver in around 11–75% and 20–40% of patients, respectively (3-5). Sometimes, in selected RCC patients with few active metastases an oligometastatic state or oligoprogression persist after previous radical resection of primary tumor and administration of systemic therapy (ST). This category of patients with slow disease progress and long survival may benefit from aggressive local therapies such as surgery and stereotactic body radiotherapy (SBRT) to the active sites of disease as suggested also by National Comprehensive Cancer Network (NCCN) guidelines version 3.2019 (6). Traditionally, metastasectomy in association or not to ST have been a standard treatment in patients with good prognosis (7). Five-year overall survival (OS) is variable and ranges from 30–58% in patients with favorable predictive factors (solitary metastasis, complete metastasectomy, presence of pulmonary metastases, good performance status) to 2–11% in patients with unfavorable factors such as incomplete resection of the metastasis or shorter distant disease-free interval (<2 years) (8-12). However, there are conflicting results among different studies regarding prognostic factors that could potentially relate to survival and local control. An effective improvement of combined therapy including targeted therapy associated to radical surgery in limited stage IV disease remains challenging and still to be demonstrated (13,14).
In the oligometastatic setting, patients are often unsuitable to invasive surgery due to comorbidities or to larger or technically not accessible metastases that may be complex to resect completely without associated morbidity (15). Therefore, SBRT could offer some advantages such as the ability to provide a less invasive ablative option of cure, obtaining at the same time very good local control and low rates of toxicity. At present, there is limited data on large series of RCC oligometastatic patients treated with SBRT because many patients, unfit to surgery, are considered palliative cases and undergo ST and/or palliative radiotherapy (RT). A recent retrospective study by Altoos et al. (16) evaluated radiographic response rate for 53 thoracic, abdominal, skin and soft tissue RCC metastases after SBRT (25–50 Gy delivered in 1–10 fractions) compared to conventional fractionated RT (20–55 Gy). After a median follow-up of 16 months, local control at 24-month was significantly greater and more durable in patients receiving SBRT vs. conventional fractionated RT (93% vs. 35%, P<0.001).
Targeted therapy is usually administered in patients with distant disease at diagnosis or further progression after radical nephrectomy (17). A recent retrospective analysis investigated the therapeutic strategy from start of first-line therapy in RCC patients with oligoprogression that presented metastases manly in the lung, liver, contralateral kidney and bone (18). Local therapy such as surgery, RT or ablative techniques in the site of progression combined to continued targeted therapy was associated to longer median post-progression OS. Older data reported a very high local control of treated metastases of about 98% in RCC oligometastatic patients but they were analyzed together with primary RCC or other primary tumors (2,19,20). The latter studies were also limited by the use of an outdated stereotactic technique. Instead, other more recent studies using advanced technique suggest that larger fractions or higher total dose as well as a high effective biological dose (BED) may improve outcomes (16,21). Considering this background, recent studies with large and homogeneous cohorts are necessary to extend the current knowledge on this topic.
In the recently published study by Franzese et al. (22), local control and progression-free survival (PFS) rates at 18-month were 90.2% and 35%, respectively, while severe treatment-related toxicities were not observed. The authors aimed to analyze outcomes in terms of disease control and survival, and side effects. In addition, independent variables were considered to find relevant prognostic factors. Eligible patients were those with previously resected primary tumor, a maximum of three distant metastases and at the same time not suitable for radical surgery due to unfavorable location or size of the metastases, or poor clinical condition of the patients and/or comorbidities. Almost two third of the patients (65.5%) received previous ST [mostly with tyrosine kinase inhibitors (TKI) in 46.5% of the cases], while the remaining 34.5% of the patients had no ST. RT was performed with stereotactic technique and median total dose was 45 Gy (range, 18–75 Gy) delivered in median 5 fractions (range, 1–10 fractions). Tumor was contoured as clinical target volume (CTV) and median diameter was 26 mm. A 5–10 mm isotropic margin was added to the CTV to determine a planning target volume (PTV) that had a median diameter of 39 mm. The data were retrospectively collected in 58 patients with 73 oligometastases from RCC treated with SBRT between 2004 and 2016. Mean age was 66 years and the most common histology was clear cell (82.7%). Forty-six (79.3%) patients had metachronous metastases. Lung was the most frequent site of oligometastases (53.4%), followed by lymph nodes (26%), bone (9.5%), adrenal gland (6.8%) and liver (4.1%). Median time to SBRT was 55 months and median follow-up after SBRT was 16.1 months. Overall, treatment was well-tolerated with no grade ≥3 acute or late toxic effects and very low rates of grade ≤2 toxicity. Median local control rate at 18-month was 90.2% and median time to local relapse was not reached. Median PFS and OS were 11.1 and 28.4 months, respectively. On multivariable analysis, metachronous and single site metastases predicted for better PFS (P=0.001; P=0.002). Moreover, local progression of oligometastases after SBRT related significantly to poorer PFS (P=0.020). A stratified analysis in patients with clear cell subtype showed that OS improved significantly when TKI or intraosseous infusion therapy was administered compared to standard chemotherapy or no ST before SBRT (P=0.019). This study is limited by the retrospective nature and the small series of patients but on the other hand adds further evidence in favor of SBRT in the treatment of oligometastatic RCC patients (see Table 1).
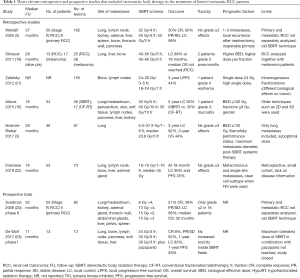
Full table
Historically, RCC has been considered a radioresistant tumor but high radiation doses with BED >100 Gy could overcome this problem by offering other radiobiological models not yet sufficiently explored. Furthermore, high dose delivered in a few fractions could guarantee good local control as already demonstrated by the current retrospective study (22). SBRT treatment could be used in oligometastatic patients who are not candidates for radical surgery or as a non-invasive option of therapy. In patients with oligoprogression, the use of SBRT in active sites not responding to ST may even delay the start of a second line therapy. Since SBRT is a well-tolerated treatment, it would be useful to compare toxicity and morbidity with surgical series, considering also local control and distant disease control. Due to lack of randomized and prospective data, retrospective studies with large and homogeneous series of patients could play an important role giving recommendations in clinical practice. It would be interesting to evaluate SBRT also in association with systemic therapies such as targeted therapy.
In conclusion, SBRT could be considered an important option of radical local therapy for the management of limited metastatic RCC patients by offering a good disease control and tolerance. Although, the most effective doses and fractionation have still to be found. This approach should be investigated in larger prospective controlled trials to confirm which subgroups of patients are most likely to benefit. Selection criteria of the candidates and association to ST are still to be defined.
Acknowledgments
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Lipworth L, Morgans AK, Edwards TL, et al. Renal cell cancer histological subtype distribution differs by race and sex. BJU Int 2016;117:260-5. [Crossref] [PubMed]
- Wersäll PJ, Blomgren H, Lax I, et al. Extracranial stereotactic radiotherapy for primary and metastatic renal cell carcinoma. Radiother Oncol 2005;77:88-95. [Crossref] [PubMed]
- Hoerner-Rieber J, Duma M, Blanck O, et al. Stereotactic body radiotherapy (SBRT) for pulmonary metastases from renal cell carcinoma—a multicenter analysis of the German working group “Stereotactic Radiotherapy”. J Thorac Dis 2017;9:4512-22. [Crossref] [PubMed]
- Kyoda Y, Kobayashi K, Hirobe M, et al. Evaluation of long-term outcome for patients with renal cell carcinoma after surgery: analysis of cancer deaths occurring more than 10 years after initial treatment. Int J Clin Oncol 2014;19:146-51. [Crossref] [PubMed]
- Grimes NG, Devlin JM, Dunne DF, et al. A systematic review of the role of hepatectomy in the management of metastatic renal cell carcinoma. Eur J Surg Oncol 2014;40:1622-8. [Crossref] [PubMed]
- National Comprehensive Cancer Network Clinical Practice Guideline in Oncology. Kidney Cancer Version 3. 2019.
- Daliani DD, Tannir NM, Papandreou CN, et al. Prospective assessment of systemic therapy followed by surgical removal of metastases in selected patients with renal cell carcinoma. BJU Int 2009;104:456-60. [Crossref] [PubMed]
- Kierney PC, van Heerden JA, Segura JW, et al. Surgeon's role in the management of solitary renal cell carcinoma metastases occurring subsequent to initial curative nephrectomy: an institutional review. Ann Surg Oncol 1994;1:345-52. [Crossref] [PubMed]
- Kavolius JP, Mastorakos DP, Pavlovich C, et al. Resection of metastatic renal cell carcinoma. J Clin Oncol 1998;16:2261-6. [Crossref] [PubMed]
- van der Poel HG, Roukema JA, Horenblas S, et al. Metastasectomy in renal cell carcinoma: A multicenter retrospective analysis. Eur Urol 1999;35:197-203. [Crossref] [PubMed]
- Kwak C, Park YH, Jeong CW, et al. Metastasectomy without systemic therapy in metastatic renal cell carcinoma: comparison with conservative treatment. Urol Int 2007;79:145-51. [Crossref] [PubMed]
- Renaud S, Falcoz PE, Alifano M, et al. Systematic lymph node dissection in lung metastasectomy of renal cell carcinoma: an 18 years of experience. J Surg Oncol 2014;109:823-9. [Crossref] [PubMed]
- Tornberg SV, Visapää H, Kilpeläinen TP, et al. Surgery for metastases of renal cell carcinoma: outcome of treatments and preliminary assessment of Leuven-Udine prognostic groups in the targeted therapy era. Scand J Urol 2018;52:419-26. [Crossref] [PubMed]
- Li JR, Ou YC, Yang CK, et al. The Impact of Local Intervention Combined with Targeted Therapy on Metastatic Renal Cell Carcinoma. Anticancer Res 2018;38:5339-45. [Crossref] [PubMed]
- Kothari G, Louie AV, Pryor D, et al. Stereotactic body radiotherapy for primary renal cell carcinoma and adrenal metastases. Chin Clin Oncol 2017;6:S17. [Crossref] [PubMed]
- Altoos B, Amini A, Yacoub M, et al. Local Control Rates of Metastatic Renal Cell Carcinoma (RCC) to Thoracic, Abdominal, and Soft Tissue Lesions Using Stereotactic Body Radiotherapy (SBRT). Radiat Oncol 2015;10:218. [Crossref] [PubMed]
- Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med 2017;376:354-66. [Crossref] [PubMed]
- Santini D, Ratta R, Pantano F, et al. Outcome of oligoprogressing metastatic renal cell carcinoma patients treated with locoregional therapy: a multicenter retrospective analysis. Oncotarget 2017;8:100708-16. [Crossref] [PubMed]
- Stinauer MA, Kavanagh BD, Schefter TE, et al. Stereotactic body radiation therapy for melanoma and renal cell carcinoma: impact of single fraction equivalent dose on local control. Radiat Oncol 2011;6:34. [Crossref] [PubMed]
- Svedman C, Sandström P, Pisa P, et al. A prospective Phase II trial of using extracranial stereotactic radiotherapy in primary and metastatic renal cell carcinoma. Acta Oncol 2006;45:870-5. [Crossref] [PubMed]
- Zelefsky MJ, Greco C, Motzer R, et al. Tumor control outcomes after hypofractionated and single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases from renal cell carcinoma. Int J Radiat Oncol Biol Phys 2012;82:1744-8. [Crossref] [PubMed]
- Franzese C, Franceschini D, Di Brina L, et al. Role of Stereotactic Body Radiation Therapy for the Management of Oligometastatic Renal Cell Carcinoma. J Urol 2019;201:70-6. [Crossref]
- De Wolf K, Rottey S, Vermaelen K, et al. Combined high dose radiation and pazopanib in metastatic renal cell carcinoma: a phase I dose escalation trial. Radiat Oncol 2017;12:157. [Crossref] [PubMed]


