
Postobstructive pneumonia in lung cancer
Introduction
In the United States, more than 200,000 cases of lung and bronchus cancer are reported each year, along with an estimate of over 150,000 deaths (1). In patients with lung cancer, age and advanced stage of the cancer increase the risk of infection. In patients with an initial diagnosis of pneumonia, a diagnosis of lung cancer has been noted to be more frequent in patients older than 65 years and in those with nonresolving pneumonia (2).
Pneumonia has been reported to occur in 50–70% of patients with lung cancer (3). Derangements at the level of the immune system and lung architecture make patients with lung cancer more susceptible to infections. Changes in immunity include the immunocompromise from the malignancy itself and from side effects of the different treatment modalities used to treat it. Changes in lung architecture include structural abnormalities such as airway obstruction. Postobstructive pneumonia is defined as an infection of lung parenchyma secondary to bronchial obstruction (4). It is often associated with lung malignancy (see Figure 1). The first documented cases were described in 1949 by McDonald et al., who defined it as a radiographic opacity resulting from complete or partial obstruction of the airway by a lung tumor (5). Bronchial obstruction is more common with tumors arising centrally, such as small cell lung carcinoma (SCLC) and squamous cell carcinoma (SCC) (6). Airway obstruction has also been seen with carcinoid tumors, which usually have a more indolent presentation (7). Neoplasms that metastasize to the lungs such as carcinomas of the breast, ovaries, colon and kidney can also cause obstructive atelectasis, leading to poor airway clearance, microbial airway colonization and eventually pneumonia (8). Other malignancies with a propensity to cause airway obstruction include lymphomas and tumors of the neck, thyroid, larynx and esophagus (9).
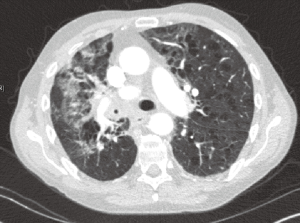
Postobstructive pneumonia can present at different stages of lung cancer and may occasionally be the initial manifestation of a malignancy (10). However, most cases are associated with advanced neoplasms. Up to 50% of patients with advanced lung cancer and pneumonia will have endobronchial compromise. This coexistence is associated with significantly increased morbidity and mortality (11). Pneumonia secondary to malignant obstruction is usually polymicrobial and difficult to eradicate with antibiotics alone (12). Infection distal to such obstructions can progress to complications like lung abscess, empyema or fistula formation in up to 10–15% of patients (4).
Besides the traditional treatments for cancer (surgery, chemotherapy and radiotherapy), additional therapeutic modalities are often required to overcome the obstruction. Improving endobronchial patency is usually needed to resolve such infections (13).
In this review, the key aspects of postobstructive pneumonia in lung cancer patients, including diagnostic evaluation and the most commonly used treatment modalities, will be described.
Epidemiology
Literature about postobstructive pneumonia in lung cancer patients is somewhat limited. Some reports suggest that 2–5% of cases of community acquired pneumonia are caused by an obstruction proximal to the site of infection, with an underlying malignancy in most of these cases. The majority of these patients also have a history of smoking, which further increases the risk of malignancy (14). Abers et al. reported how malignancy was discovered to be the cause of the obstruction in approximately 50% of patients with postobstructive pneumonia (15).
Postobstructive pneumonia, however, is more common in patients with established lung cancer. About 45–50% of patients with advanced or rapidly progressing cancer will have a postobstructive pneumonia at some point (4). Other authors report that up to 80% of non-resectable lung cancers will manifest evidence of airway obstruction during the course of their disease (16,17).
Pathogenesis
The lung is a common site of infection in patients with cancer. In both, immunocompetent and immunosuppressed hosts, the initial pathophysiology of pneumonia is fairly similar and typically results from invasion and overgrowth of microorganisms in the lung parenchyma. Infectious organisms access the small airways via inhalation, aspiration, hematogenous spread or locoregional movement from larger airways. In order for bacteria to cause infection, they have to breach the patient’s complex array of dynamic defense apparatuses that include architectural, cellular and humoral mechanisms (18). In immunocompetent patients, these defense systems are usually intact and capable of eliminating pathogens.
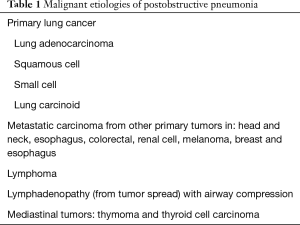
Multiple types of malignancies have been associated to postobstructive pneumonia (see Table 1). Along with the mechanical consequences of cancer-related airway obstructions, lung cancer patients frequently have a wide variety of immune defects, which increase their likelihood of developing lung infections. These changes in immunity are caused by both the malignancy and its treatment, including surgery, radiotherapy and chemotherapy (19). Lee et al. have described how the general debility from cancer and its treatment strongly influence the incidence of lung infections. An Eastern Cooperative Oncology Group (ECOG) score ≥2 has been identified as a risk factor for pneumonia. Nutritional deficiencies, commonly seen in patients with malignancy, typically have a deleterious effect (20). Other underlying lung diseases, such as chronic obstructive pulmonary disease (COPD), emphysema and bronchiectasis, commonly found in patients with lung cancer and smoking history, also increase the incidence of infections by affecting innate anatomical defense mechanisms resulting in poor clearance of secretions and bacteria.
Full table
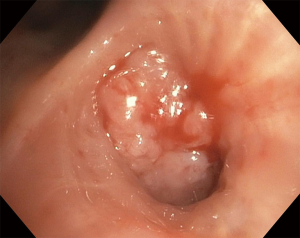
Luminal airway obstruction due to malignancy can be due to different mechanisms, including extrinsic compression by a tumor or enlarged lymph nodes, endobronchial obstruction from an intraluminal mass (see Figure 2) or a combination of both (21). Smaller airways can get obstructed by lymphangitic spread of malignancy (22). These mechanisms have in common the stasis of secretions in bronchi and alveoli distal to the obstruction, formation of atelectasis and subsequent microbial colonization and infection (23). Established infections can cause extensive local destruction of the lung parenchyma, occasionally complicated by formation of lung abscess or empyema, often requiring invasive treatment modalities in addition to the use of antimicrobials (3).
Cancer treatments, such as chemotherapy, radiotherapy and surgery can also cause derangements of innate and adaptive immune defense mechanisms responsible for protecting the lungs from infections. Chemotherapy often leads to neutropenia, which when present, particularly with counts lower than 500 cells/µL, is associated with severe infections and poor outcomes. Functional neutropenia secondary to radiation, steroids and hyperglycemia also contributes to increased risk of pneumonia by impairing the phagocytic and chemotactic properties of neutrophils (24). Radiation and surgery can cause airway distortion and the risk of introducing microorganisms during surgical procedures. Presence of endobronchial stents used to open airways affected by extrinsic compression from lung tumors can also precipitate lung infections by different mechanisms, including the introduction of pathogens during the procedure or the colonization and growth of microorganisms on the stent (25).
Microbiology
There are not many studies describing the microbiology of postobstructive pneumonia in lung cancer patients, but those available have shown infections with a wide spectrum of microorganisms (see Table 2). The variability is particularly dependent on the immunologic defects related to the underlying neoplasm and the various treatment modalities that the patient has received (26). Bacterial, viral and fungal infections often coexist in these patients (27).

Full table
In patients with untreated cancer who are relatively non-immunocompromised, the predominant microorganisms are pretty similar to those affecting non-cancer patients with community acquired pneumonia. These include Streptococcus pneumonia, Haemophilus influenza, Moraxella catarrhalis and some viruses. Studies involving patients with advanced lung cancer that have received treatment, leading to multifactorial immunosuppression, describe a preponderance of other organisms such as Pseudomonas aeruginosa, Staphylococcus aureus, Enterobacter cloacae and Acinetobacter species (28). Oral anaerobes such as Bacteroides, Prevotella, Fusobacterium and Actinomyces have also been isolated from postobstructive pneumonias.
Once cellular immunity has been impaired, other viral infections such as cytomegalovirus and herpes can coexist with bacterial infections. Fungal organisms like Candida, Aspergillus, Histoplasma and Coccidioides cause pulmonary infections in these patients as well. Pneumocystis jirovecii pneumonia is also seen in this setting. Impaired humoral immunity increases susceptibility to infections by encapsulated organisms. In neutropenic patients with postobstructive pneumonia, the spectrum of infection has shifted from a predominance of gram-negative bacilli to one of gram-positive cocci, particularly Staphylococcus (including methicillin-resistant Staphylococcus aureus), Streptococcus and Enterococcus. In persistent neutropenia, superinfection with multidrug resistant (MDR) gram-negative organisms (Pseudomonas, Stenotrophomonas, Acinetobacter and Citrobacter) and fungi (Aspergillus, Zygomycetes and Fusarium species) is common.
Certain microorganisms have been found to be more commonly associated with complications. For example, the most commonly organisms seen in empyema include Staphylococcus aureus and gram-negative bacilli.
Microbiologic specimens, unfortunately, are difficult to obtain since infection is typically distal to the area of obstruction (29). Hsu-Kim et al. have described how the diagnostic yield is significantly increased by performing bronchoalveolar lavage (BAL) and needle aspiration (12).
Clinical presentation
The classic symptoms of postobstructive pneumonia are similar to that of any pneumonia. Fever, cough and dyspnea are present in the majority of cases. Cough is productive and purulent unless there is complete obstruction. Loss of appetite and weight loss may be due to the malignancy and is worsened by the development of pneumonia. Cachexia can be seen in >50% of cases. Hemoptysis and chest pain are not as common. Sometimes carcinoid tumors, especially if central, will present with hemoptysis, wheezing and bronchial obstruction. A proximal partial occlusion can manifest itself as localized monophonic wheezing.
The constellation of signs and symptoms also depends on whether the postobstructive pneumonia is diagnosed in the setting of a known advanced lung malignancy or if a non-resolving pneumonia led to the discovery of an underlying cancer. COPD is a common comorbidity in patients with postobstructive pneumonia in the setting of lung cancer and thus such a patient can present as an acute exacerbation of COPD with pneumonia.
In a prospective study, patients with postobstructive pneumonia due to malignancy, when compared to patients with bacterial pneumonia, had a longer duration of symptoms (14 vs. 5 days) and were more likely to have weight loss and cavitary lesions but less likely to have leukocytosis (15).
Evaluation
Postobstructive pneumonia is often initially described as “nonresolving pneumonia” and its evaluation generally requires a detailed workup. The presence of an underlying malignancy should be suspected based solely on the clinical presentation. Suspicion should prompt the use of more advanced tools to look for an extrinsic or intrinsic airway occlusion as well as to obtain microbiologic and pathologic specimens. Imaging and bronchoscopy play key roles in diagnosing postobstructive pneumonia.
Pneumonia can be the first manifestation of a malignancy, and so a prompt diagnosis can be challenging unless a high degree of suspicion is maintained when evaluating such patients.
Imaging
Initial evaluation is typically with a chest radiograph, which is easily available and noninvasive (30). Its main disadvantage is its low specificity and poor negative predictive value. In addition, radiographic evaluation has been shown to have lower detection rates in immunocompromised patients with pneumonia (31). On plain radiographs, it is often difficult to differentiate tumor from postobstructive consolidation or atelectasis. However, it is helpful in determining when a patient has a “nonresolving pneumonia”. In 1991, Kirtland and Winterbauer radiologically defined “slowly resolving pneumonia” as clearing of radiographic infiltrate <50% in 2 weeks or incomplete clearing at 4 weeks in a patient who has clinically responded to antibiotics (32). Other authors have defined it more broadly as radiographic infiltrate that is slow to resolve after optimal antibiotic therapy given for at least 10 days (33).
CT of the chest is not only more sensitive than plain radiography in detecting and characterizing of pneumonia (see Figure 1), particularly when intravenous (IV) contrast is used or when performed with high-resolution formatting (34). It helps determine the possible site of obstruction, distinguish consolidation from neoplasm or lymphadenopathy and evaluate the patency of distal bronchi, the relationship between mass and adjacent structures and the extent of pleural involvement (35,36).
New generation CT scanners, by acquiring thinner cuts, create better quality images and can generate a variety of two-dimensional multiplanar and three-dimensional reconstructions. These help direct bronchoscopic interventions that are aimed not only at obtaining diagnostic samples, but also to relieve the obstruction (37,38).
Ultimately though, fiberoptic bronchoscopy plays the central role in making a diagnosis of airway obstruction and malignancy (39). Better sputum samples can be obtained by protected specimen brushing (PSB) and BAL which is the diagnostic tool of choice (40).
Endobronchial ultrasound by identifying a lung mass or enlarged lymph node, followed by fine needle aspiration and/or transbronchial biopsy, is often invaluable in the diagnosis of a neoplasm and determining the extent of involvement.
Treatment
The management of postobstructive pneumonia is initially dependent on appropriate antibiotic therapy but will eventually require relief of the obstruction. The presence of the obstruction proximal to the infection predisposes to slow or even incomplete response to antibiotics, leading to frequent recurrence of the infection. Repeated treatments can then create significant resistance to antimicrobials (41). The duration of antibiotic regimen is usually more prolonged than pneumonia not associated with malignant obstruction and can last for 6 weeks or more.
Empiric broad spectrum antibiotics are usually selected initially since, at the initial evaluation, it is difficult to obtain information about the specific infection present distal to the obstruction. Antimicrobial regimens are typically guided by local prevalence and resistance patterns among MDR pathogens (41).
Response to antimicrobial therapy can be delayed and, therefore, it is not uncommon to observe persistence or recurrence of the pneumonia. If the infection progresses, it can lead to complications with serious consequences such as lung abscess, hemorrhage, empyema and formation of bronchopleural fistula (41). Estimated incidence of these complications is in the range of 10–15%. Due to their mixed bacterial etiology, their treatment is more challenging. Additionally, these complications can prevent prompt initiation of chemotherapy, thus further delaying resolution of the obstruction.
If the lesion is contained, surgery is usually the treatment of choice. If curative resection is not an immediate option, treatment should be attempted with one of several invasive methods available, individualizing decisions in each case. Interventional techniques allow treatment of the obstruction to facilitate drainage of secretions. With advances in interventional pulmonology, endobronchial treatment has become a safe, effective and important tool in the management of these patients.
Pleural effusion associated with ipsilateral malignancy and obstructive pneumonia can be paramalignant and transudative due to bronchial obstruction or parapneumonic and exudative secondary to pneumonia. Progressive pneumonia can lead to an infected pleural space and empyema. Management of the effusion includes evaluation of the pleural fluid and treatment of the obstruction. Manometry when available should be combined with the thoracentesis to evaluate for lung entrapment.
Fluid should be subjected to a thorough chemical, microbiological and cytological analysis. Benign transudative effusions can be managed by relief of bronchial obstruction and therapeutic thoracentesis. Small bore chest tube drainage, intra-pleural fibrinolytic therapy and decortication may be required for parapneumonic effusions or empyema. Surgical resection when undertaken in the presence of an infected pleural space carries a higher risk of bronchopleural fistula if the bronchial stump gets infected.
Tunneled pleural catheters combined with pleurodesis are indicated for confirmed malignant pleural effusions. Of note, application of talc under direct visualization achieves superior results compared to pleural catheter-directed application.
External beam radiotherapy (EBRT) for airway obstruction
Patients with advanced lung cancer, airway obstruction and poor performance status might not be suitable candidates for more invasive interventions. When immediate management of the airway is required, EBRT can be considered an alternative (42,43). Lee et al. evaluated the response to EBRT in 95 patients with obstructed airways due to different types of lung cancer. Not only did they find that EBRT was effective in resolving airway obstruction (reported response rate of 78.9%), but they also described a significant increase in 1-year survival rate in these patients. The type of tumor did not affect response to treatment but response was significantly better in tumors <6 cm (43). Application of local radiotherapy for 10–12 days at doses of 30–40 Gy have resulted in palliation of lung mass symptoms, including relief of obstruction. When compared to endobronchial brachytherapy, ERBT showed better outcomes (44).
Interventional modalities to relieve obstruction
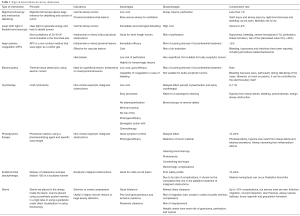
Interventional pulmonology also offers different alternatives to manage external obstructions and to treat endobronchial lesions (45), leading to relief of obstruction, palliation of symptoms and treatment of infection. Because this condition is more commonly seen in advanced malignancy and in patients with a higher operative risk due to their poor pulmonary reserve, deconditioning and increased use of steroids, bronchoscopic procedures often become the preferred therapeutic option (17). There are multiple modalities available, including endobronchial laser therapies, brachytherapy, argon plasma coagulation (APC), cryotherapy, electrocautery, photodynamic therapy (PDT), airway dilatation and stent placement (46). Mechanical debulking and thermal techniques are usually preferred for intraluminal tumors, while stents are the preferred method to treat extrinsic obstructions. See summary of techniques in Table 3.
Full table
Laforet et al. first described the use of laser for the treatment of airway tumors in 1976 (47). Laser can be applied using both flexible and rigid bronchoscopes (17). This therapy targets endobronchial and endotracheal obstructions, enabling tumor destruction and appropriate hemostasis when needed (48). Laser has been shown to be beneficial to treat obstructions in proximal airways, ideally with a short length of involvement (<4 cm) and minimal submucosal infiltration. Treatment of obstructions in segmental bronchi is more difficult (49). Laser has been found to provide quick and meaningful improvement in symptoms, airway patency, hypoxemia, pulmonary function and quality of life when used for endobronchial obstructions (50). It has also often led to significant improvement in the drainage of mucus and resolution of the infectious process (51). Complications of laser are rare (around 2–3%) when the procedure is done by a trained operator, with procedural deaths less than 1%. Complications include hypoxemia, bleeding (severe hemoptysis reported in about 1% of cases), perforation, fistula formation and fire. The risk of fire can be decreased by using low inspired oxygen concentrations, not higher than 40% (52).
APC is a non-contact electrocoagulation that uses heated argon plasma as a conducting medium, offering a more uniform distribution of its therapeutic effect with less manipulation and trauma to the target tissue. This technique devitalizes tissue with high temperatures, coagulating and desiccating tissue. It is a good option for managing distal or extensively hemorrhagic tumors (53). It is also a good option for lesions that measure less than 3.5 cm in length that protrude within the lumen and do not extend beyond the cartilage of the airway (54). Complications rates are low given its superficial effect. Major complications occur in less than 5% of cases (55).
Electrocautery is the application of electric current to deliver thermal energy resulting in tissue destruction. At low power it can lead to coagulation and at high power it causes vaporization or carbonization. It can coagulate lesions, which can later be removed by suction or forceps. It is ideal for superficial coagulation and provides rapid results with relatively low risk of airway perforation. While responses are often similar, electrocautery is more economical than modalities such as laser, brachytherapy and cryotherapy. In case of endobronchial compromise by an extrinsic tumor, placement of a stent is usually considered complementary to thermocoagulation (56). Rates of complications with electrocautery are low, especially with the soft coagulation mode although scarring stenosis has been described, particularly after application of circumferential thermocoagulation (53).
Endobronchial brachytherapy involves applying radioactive isotopes (Iridium 192) in a highly localized manner to the tumor. It is usually given using a catheter under direct visualization with bronchoscopy. This allows preservation of surrounding healthy tissue (57). It can be used to treat lesions with endobronchial infiltration. Its use can improve symptoms and functional parameters. However, there is a significant rate of severe complications (reportedly as high as 10–20%) (58). Massive hemoptysis is one of the most feared complications and can occur in approximately 7% of patients. Other complications are radiation bronchitis, abscess formation, bronchial wall necrosis and esophago-bronchial fistula formation (59-61).
Cryotherapy uses very low temperature, causing dehydration and cellular crystallization of tissues in the short term and apoptosis and ischemia in the long term. It is useful in bleeding lesions especially when the exact site of hemorrhage is not known (62,63). Overall mortality is low (1–2%) with complication rates <10% (64) Cryotherapy has a favorable cost and risk benefit ratio and rarely causes stenosis secondary to scarring. Delayed effects give an additional benefit. Another advantage of cryotherapy is that it can be delivered near stents without damaging them.
PDT causes tumor destruction by activating a photosensitizing agent using a light source with a specific wavelength, depending on the agent selected for each case. This induces a phototoxic reaction and cell death (65). It can achieve marked, yet delayed, symptom improvement and is, therefore, not suitable for critical proximal obstructions (66,67). Hemoptysis has been reported as side effect (67). Since the collagen remains unaffected, the risk of perforation is almost nonexistent (68).
Airway dilatation or bronchoplasty can be achieved by different modalities, including rigid bronchoscopy, semi-rigid dilators and balloon dilators. Dilation of airway stenosis by rigid bronchoscopy is becoming less common, being replaced by flexible bronchoscopy and balloon dilatation, which has the advantage of producing less mucosal trauma and consequent granulation tissue. It also has an immediate effect of relieving extrinsic and intrinsic obstructions, sometimes preceding the insertion of a stent or the use of other interventions such as laser, electrocautery or APC, particularly if there is increased risk of airway restenosis. This can have an impact on the treatment of postobstructive pneumonia by further improving clearance of secretions. Hautmann et al. did a prospective study where 78 patients with malignant airway obstruction underwent bronchoscopic balloon dilatation (BBD) and an immediate improvement was reported in 79% of patients (69).
Stents (silicone or covered) are used to establish airway patency by counteracting extrinsic compression from a tumor or enlarged lymph nodes. They are also a complementary method after endobronchial debulking of a tumor. No clear advantage of one kind of stent over another has been described. After stent placement, patients usually have an improvement in their quality of life and pulmonary function. Some patients that suffered respiratory failure due to a malignant tracheobronchial lesion have been weaned off mechanical ventilation soon after implantation of a self-expandable stent under flexible bronchoscopy.
Combination of techniques
Depending on the mechanism of stenosis, various bronchoscopic techniques can be chosen from and performed alone or in combination. Some techniques are non-complementary like laser and thermocoagulation while other techniques can be complementary. An example of the latter is debulking followed by stent placement. Laser with its immediate effect can be synergistic with brachytherapy and data supports good symptomatic relief and extended survival (60% with combination vs. 30% survival at 7 months with laser only). Brachytherapy is marred by a high complication rate and that is the biggest hurdle in using the laser-brachytherapy combination widely. Cryotherapy can be used to desiccate the base of an intraluminal tumor following which mechanical debulking, laser or electrocautery can further improve the airway lumen.
The goal of all these techniques is to obtain the greatest relief of the airway obstruction as quickly as possible in order to palliate symptoms and to contribute to the treatment of a postobstructive pneumonia if present (see Figure 3).
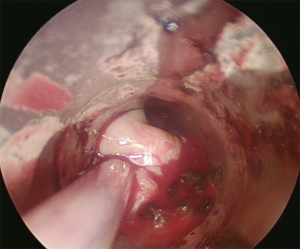
Outcomes and prognosis
Postobstructive pneumonia significantly increases morbidity and mortality, particularly in advanced lung cancer. Its treatment often requires multiple long courses of antibiotics, thus contributing to the development of resistant organisms. The literature on the prognosis of postobstructive pneumonia is scant and is probably largely dependent on the primary malignancy and its stage. The response of the obstruction to interventional techniques is variable and exposes the patient to the risk of anesthesia, procedural complications and nosocomial infections.
Conclusions
Post-obstructive pneumonia in patients with advanced lung malignancy is a serious, often life-threatening development that occurs in approximately 50% of patients. Diagnosis of postobstructive pneumonia can be challenging. In some cases, infiltrates and consolidations distal to a lung tumor might not be infectious. Nevertheless, almost all patients are treated with broad-spectrum antimicrobial regimens and, when infectious, seldom resolve completely. Recurrent or refractory infections are common, and other serious complications such as lung abscess, empyema, and fistula formation are also seen in this setting leading to rapid clinical deterioration.
Interventions to overcome obstruction are based on the nature of the obstruction, available techniques, quality of life concerns and physician expertise. No one modality is clearly superior to the others, and often multiple modalities need to be used in the same patient. In addition to relieving airway obstruction which will hasten resolution and prevent recurrence of infections, it is also helpful in decompensated patients by improving oxygenation and ventilation. If the stage of the malignancy is advanced, the primary goal is to improve quality of life and secondarily to potentially prolong survival.
Acknowledgments
All images in this review are courtesy of Dr. Kassem Harris, Interventional Pulmonology, Westchester Medical Center, Valhalla, NY. He authorized its use for this article.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Marrie TJ. Pneumonia and carcinoma of the lung. J Infect 1994;29:45-52. [Crossref] [PubMed]
- Akinosoglou KS, Karkoulias K, Marangos M. Infectious complications in patients with lung cancer. Eur Rev Med Pharmacol Sci 2013;17:8-18. [PubMed]
- Rolston KV. Postobstructive Pneumonia in Cancer Patients. Clin Infect Dis 2016;63:707-8. [Crossref] [PubMed]
- McDonald JR, Harrington SW, ClagetT OT. Obstructive pneumonitis of neoplastic origin; an interpretation of one form of so-called atelectasis and its correlation according to presence of absence of sputum. J Thorac Surg 1949;18:97-112; disc., 122.
- Kalkat MS, Bonser RS. Obstructive pneumonia: an indication for surgery in mega aorta syndrome. Ann Thorac Surg 2003;75:1313-5. [Crossref] [PubMed]
- Waheed Z, Irfan M, Fatimi S, et al. Bronchial carcinoid presenting as multiple lung abscesses. J Coll Physicians Surg Pak 2013;23:229-30. [PubMed]
- Kohno S, Koga H, Oka M, et al. The pattern of respiratory infection in patients with lung cancer. Tohoku J Exp Med 1994;173:405-11. [Crossref] [PubMed]
- Mohapatra PR, Bhuniya S, Garg S, et al. Endobronchial non-Hodgkin's lymphoma presenting as mass lesion. Indian J Chest Dis Allied Sci 2009;51:107-9. [PubMed]
- Yang PC, Luh KT, Wu HD, et al. Lung tumors associated with obstructive pneumonitis: US studies. Radiology 1990;174:717-20. [Crossref] [PubMed]
- Torres A, Ferrer M. Editorial Commentary: Distinguishing Postobstructive Lung Infection from Community-Acquired Pneumonia. Clin Infect Dis 2016;62:962-3. [Crossref] [PubMed]
- Hsu-Kim C, Hoag JB, Cheng GS, et al. The microbiology of postobstructive pneumonia in lung cancer patients. J Bronchology Interv Pulmonol 2013;20:266-70. [Crossref] [PubMed]
- Murgu SD, Egressy K, Laxmanan B, et al. Central Airway Obstruction: Benign Strictures, Tracheobronchomalacia, and Malignancy-related Obstruction. Chest 2016;150:426-41. [Crossref] [PubMed]
- Woodhead MA, Macfarlane JT, McCracken JS, et al. Prospective study of the aetiology and outcome of pneumonia in the community. Lancet 1987;1:671-4. [Crossref] [PubMed]
- Abers MS, Sandvall BP, Sampath R, et al. Postobstructive Pneumonia: An Underdescribed Syndrome. Clin Infect Dis 2016;62:957-61. [Crossref] [PubMed]
- Ernst A, Silvestri GA, Johnstone D. Interventional pulmonary procedures: Guidelines from the American College of Chest Physicians. Chest 2003;123:1693-717. [Crossref] [PubMed]
- Mehta RM, Cutaia M. The role of interventional pulmonary procedures in the management of post-obstructive pneumonia. Curr Infect Dis Rep 2006;8:207-14. [Crossref] [PubMed]
- Evans SE, Xu Y, Tuvim MJ, et al. Inducible innate resistance of lung epithelium to infection. Annu Rev Physiol 2010;72:413-35. [Crossref] [PubMed]
- Madenspacher JH, Azzam KM, Gowdy KM, et al. p53 Integrates host defense and cell fate during bacterial pneumonia. J Exp Med 2013;210:891-904. [Crossref] [PubMed]
- Lee JO, Kim DY, Lim JH, et al. Risk factors for bacterial pneumonia after cytotoxic chemotherapy in advanced lung cancer patients. Lung Cancer 2008;62:381-4. [Crossref] [PubMed]
- Winterbauer RH, Bedon GA, Ball WC Jr. Recurrent pneumonia. Predisposing illness and clinical patterns in 158 patients. Ann Intern Med 1969;70:689-700. [Crossref] [PubMed]
- Yamada Y, Sekine Y, Suzuki H, et al. Trends of bacterial colonisation and the risk of postoperative pneumonia in lung cancer patients with chronic obstructive pulmonary disease. Eur J Cardiothorac Surg 2010;37:752-7. [Crossref] [PubMed]
- McDowell DE. Bronchoscopic detection of bronchogenic carcinoma in cases of pneumonia. South Med J 1980;73:761-2. [Crossref] [PubMed]
- Safdar A, Armstrong D. Infectious morbidity in critically ill patients with cancer. Crit Care Clin 2001;17:531-70. vii-viii. [Crossref] [PubMed]
- Noppen M, Pierard D, Meysman M, et al. Bacterial colonization of central airways after stenting. Am J Respir Crit Care Med 1999;160:672-7. [Crossref] [PubMed]
- Liaw YS, Yang PC, Wu ZG, et al. The bacteriology of obstructive pneumonitis. A prospective study using ultrasound-guided transthoracic needle aspiration. Am J Respir Crit Care Med 1994;149:1648-53. [Crossref] [PubMed]
- Aguilar-Guisado M, Jimenez-Jambrina M, Espigado I, et al. Pneumonia in allogeneic stem cell transplantation recipients: a multicenter prospective study. Clin Transplant 2011;25:E629-38. [Crossref] [PubMed]
- Nagata N, Nikaido Y, Kido M, et al. Terminal pulmonary infections in patients with lung cancer. Chest 1993;103:1739-42. [Crossref] [PubMed]
- Liao WY, Liaw YS, Wang HC, et al. Bacteriology of infected cavitating lung tumor. Am J Respir Crit Care Med 2000;161:1750-3. [Crossref] [PubMed]
- Navigante AH, Cerchietti LC, Costantini P, et al. Conventional chest radiography in the initial assessment of adult cancer patients with fever and neutropenia. Cancer Control 2002;9:346-51. [Crossref] [PubMed]
- Holmberg H, Kragsbjerg P. Association of pneumonia and lung cancer: the value of convalescent chest radiography and follow-up. Scand J Infect Dis 1993;25:93-100. [Crossref] [PubMed]
- Kirtland SH, Winterbauer RH. Slowly resolving, chronic, and recurrent pneumonia. Clin Chest Med 1991;12:303-18. [PubMed]
- Fein AM, Feinsilver SH. The approach to nonresolving pneumonia in the elderly. Semin Respir Infect 1993;8:59-72. [PubMed]
- Syrjälä H, Broas M, Suramo I, et al. High-resolution computed tomography for the diagnosis of community-acquired pneumonia. Clin Infect Dis 1998;27:358-63. [Crossref] [PubMed]
- Claessens YE, Debray MP, Tubach F, et al. Early Chest Computed Tomography Scan to Assist Diagnosis and Guide Treatment Decision for Suspected Community-acquired Pneumonia. Am J Respir Crit Care Med 2015;192:974-82. [Crossref] [PubMed]
- Mulabecirovic A, Gaulhofer P, Auner HW, et al. Pulmonary infiltrates in patients with haematologic malignancies: transbronchial lung biopsy increases the diagnostic yield with respect to neoplastic infiltrates and toxic pneumonitis. Ann Hematol 2004;83:420-2. [Crossref] [PubMed]
- Boiselle PM, Ernst A. Recent advances in central airway imaging. Chest 2002;121:1651-60. [Crossref] [PubMed]
- Finkelstein SE, Schrump DS, Nguyen DM, et al. Comparative evaluation of super high-resolution CT scan and virtual bronchoscopy for the detection of tracheobronchial malignancies. Chest 2003;124:1834-40. [Crossref] [PubMed]
- Jain P, Sandur S, Meli Y, et al. Role of flexible bronchoscopy in immunocompromised patients with lung infiltrates. Chest 2004;125:712-22. [Crossref] [PubMed]
- Sampsonas F, Kontoyiannis DP, Dickey BF, et al. Performance of a standardized bronchoalveolar lavage protocol in a comprehensive cancer center: a prospective 2-year study. Cancer 2011;117:3424-33. [Crossref] [PubMed]
- Rolston KVI, Jamal MA, Nesher L, et al. In vitro activity of ceftaroline and comparator agents against Gram-positive and Gram-negative clinical isolates from cancer patients. Int J Antimicrob Agents 2017;49:416-21. [Crossref] [PubMed]
- Nihei K, Ishikura S, Kawashima M, et al. Short-course palliative radiotherapy for airway stenosis in non-small cell lung cancer. Int J Clin Oncol 2002;7:284-8. [PubMed]
- Lee JW, Lee JH, Kim HK, et al. The efficacy of external beam radiotherapy for airway obstruction in lung cancer patients. Cancer Res Treat 2015;47:189-96. [Crossref] [PubMed]
- Stout R, Barber P, Burt P, et al. Clinical and quality of life outcomes in the first United Kingdom randomized trial of endobronchial brachytherapy (intraluminal radiotherapy) vs. external beam radiotherapy in the palliative treatment of inoperable non-small cell lung cancer. Radiother Oncol 2000;56:323-7. [Crossref] [PubMed]
- Scarlata S, Fuso L, Lucantoni G, et al. The technique of endoscopic airway tumor treatment. J Thorac Dis 2017;9:2619-39. [Crossref] [PubMed]
- Guibert N, Mazieres J, Marquette CH, et al. Integration of interventional bronchoscopy in the management of lung cancer. Eur Respir Rev 2015;24:378-91. [Crossref] [PubMed]
- Laforet EG, Berger RL, Vaughan CW. Carcinoma obstructing the trachea. Treatment by laser resection. N Engl J Med 1976;294:941. [Crossref] [PubMed]
- Squiers JJ, Teeter WA, Hoopman JE, et al. Holmium:YAG laser bronchoscopy ablation of benign and malignant airway obstructions: an 8-year experience. Lasers Med Sci 2014;29:1437-43. [Crossref] [PubMed]
- Macha HN, Becker KO, Kemmer HP. Pattern of failure and survival in endobronchial laser resection. A matched pair study. Chest 1994;105:1668-72. [Crossref] [PubMed]
- Cavaliere S, Foccoli P, Farina PL. Nd:YAG laser bronchoscopy. A five-year experience with 1,396 applications in 1,000 patients. Chest 1988;94:15-21. [Crossref] [PubMed]
- Han CC, Prasetyo D, Wright GM. Endobronchial palliation using Nd:YAG laser is associated with improved survival when combined with multimodal adjuvant treatments. J Thorac Oncol 2007;2:59-64. [Crossref] [PubMed]
- Cavaliere S, Venuta F, Foccoli P, et al. Endoscopic treatment of malignant airway obstructions in 2,008 patients. Chest 1996;110:1536-42. Erratum in: Chest. 1997;111:1476. Dosage error in article text. [Crossref] [PubMed]
- Tremblay A, Marquette CH. Endobronchial electrocautery and argon plasma coagulation: a practical approach. Can Respir J 2004;11:305-10. [Crossref] [PubMed]
- Morice RC, Ece T, Ece F, et al. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest 2001;119:781-7. [Crossref] [PubMed]
- Matveychuk A, Guber A, Talker O, et al. Incidence of bacteremia following bronchoscopy with argon plasma coagulation: a prospective study. Lung 2014;192:615-8. [Crossref] [PubMed]
- Petrou M, Kaplan D, Goldstraw P. Bronchoscopic diathermy resection and stent insertion: a cost effective treatment for tracheobronchial obstruction. Thorax 1993;48:1156-9. [Crossref] [PubMed]
- Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society/American Thoracic Society. Eur Respir J 2002;19:356-73. [PubMed]
- Mehta M, Shahabi S, Jarjour N, et al. Effect of endobronchial radiation therapy on malignant bronchial obstruction. Chest 1990;97:662-5. [Crossref] [PubMed]
- Huber RM, Fischer R, Hautmann H, et al. Palliative endobronchial brachytherapy for central lung tumors. A prospective, randomized comparison of two fractionation schedules. Chest 1995;107:463-70. [Crossref] [PubMed]
- Trédaniel J, Hennequin C, Zalcman G, et al. Prolonged survival after high-dose rate endobronchial radiation for malignant airway obstruction. Chest 1994;105:767-72. [Crossref] [PubMed]
- Hennequin C, Bleichner O, Tredaniel J, et al. Long-term results of endobronchial brachytherapy: A curative treatment? Int J Radiat Oncol Biol Phys 2007;67:425-30. [Crossref] [PubMed]
- Casal RF, Iribarren J, Eapen G, et al. Safety and effectiveness of microdebrider bronchoscopy for the management of central airway obstruction. Respirology 2013;18:1011-5. [Crossref] [PubMed]
- Marasso A, Gallo E, Massaglia GM, et al. Cryosurgery in bronchoscopic treatment of tracheobronchial stenosis. Indications, limits, personal experience. Chest 1993;103:472-4. [Crossref] [PubMed]
- Hetzel M, Hetzel J, Schumann C, et al. Cryorecanalization: a new approach for the immediate management of acute airway obstruction. J Thorac Cardiovasc Surg 2004;127:1427-31. [Crossref] [PubMed]
- Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med 2004;169:1278-97. [Crossref] [PubMed]
- Diaz-Jiménez JP, Martínez-Ballarín JE, Llunell A, et al. Efficacy and safety of photodynamic therapy versus Nd-YAG laser resection in NSCLC with airway obstruction. Eur Respir J 1999;14:800-5. [Crossref] [PubMed]
- Moghissi K, Dixon K, Thorpe JA, et al. Photodynamic therapy (PDT) in early central lung cancer: a treatment option for patients ineligible for surgical resection. Thorax 2007;62:391-5. [Crossref] [PubMed]
- Minnich DJ, Bryant AS, Dooley A, et al. Photodynamic laser therapy for lesions in the airway. Ann Thorac Surg 2010;89:1744-8; discussion 1748-9.
- Hautmann H, Gamarra F, Pfeifer KJ, et al. Fiberoptic bronchoscopic balloon dilatation in malignant tracheobronchial disease: indications and results. Chest 2001;120:43-9. [Crossref] [PubMed]


