
Bronchoscopic instillation of DNase to manage refractory lobar atelectasis in a lung cancer patient
Introduction
Lobar atelectasis is a common complication of lung cancer (1). The underlying etiology of lobar atelectasis can be due to several causes, such as tumor endobronchial invasion or external compression causing airway obstruction, as well as higher risk of lung infections and obstructive pneumonia producing copious secretions and mucus plugs (1). Typical management of lobar atelectasis focuses on treating the underlying cause and supportive care, such as chest physical therapy, bronchodilators and mucolytics and therapeutic bronchoscopy (2). However, this approach is not always effective in treating refractory atelectasis.
Dornase alfa is a recombinant human deoxyribonuclease I (rhDNase), an enzyme which selectively cleaves DNA, thus reducing mucous viscosity (3). Bronchoscopically instilled rhDNase has been reported as a rescue treatment for refractory atelectasis in newborn and pediatric populations (4-9). However, its use in adults has not been well described.
Case presentation
A 53-year-old male patient presented to the emergency department of a local hospital for abdominal pain and non-bloody emesis. The patient had an extensive medical history including congestive heart failure with reduced ejection fraction (CHFrEF), atrial fibrillation, seizure disorder, World Health Organization (WHO) group D chronic obstructive pulmonary disease (COPD) on home oxygen and obstructive sleep apnea (OSA). Patient was found to have severe hyponatremia with serum sodium level of 102 mEq/L, which was corrected to 122 mEq/L in two days. However, he developed altered mental status for which he was intubated for airway protection and transferred to our institution for suspected acute stroke vs. central pontine myelinolysis (CPM).
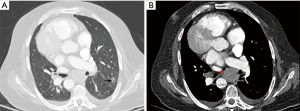
The patient was admitted to the Medical Intensive Care Unit (MICU) at our institution. His mental status improved and acute stroke was ruled out. He was successfully extubated and transferred to the medical floor the following day. However, in view of elevated liver enzymes, patient underwent hepatobiliary ultrasound which showed diffuse hypoattenuated hepatic lesions. Ultrasound guided liver biopsy revealed small cell lung carcinoma (SCLC). Further work up with full body computed tomography (CT) scan showed a right upper lobe ground nodule that has increased in size compared to a prior chest CT scan from 2011. The patient also had a new semisolid left lower lobe (LLL) nodule (16 mm × 12 mm) and diffuse exuberant lymphadenopathy throughout the thorax and abdomen (Figure 1). He was diagnosed with stage 4 SCLC based on the liver biopsy and for which he was started on chemotherapy.
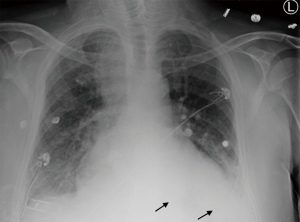
While the patient was on the medical floor, he developed shortness of breath and productive cough with green sputum. Chest X-ray (CXR) showed obscured left hemidiaphragm with right upper lobe and possible right lower lobes consolidations (Figure 2).
He was started on broad spectrum antibiotics for multi-lobar bacterial pneumonia.
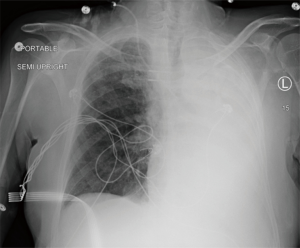
A few days later, the patient had worsened hypoxemia and was intubated. A portable CXR showed complete left lung collapse (Figure 3). He was transferred back to the MICU but had no improvement on extensive pulmonary hygiene with bronchodilators, hypertonic saline inhalation and frequent suctioning.
Hence, the patient underwent a therapeutic bronchoscopy using a therapeutic bronchoscope. There were tenacious mucoid secretions in the left lung bronchi and occlusion of the right middle lobe (RML) medial segmental bronchus by an endobronchial mass. Therapeutic aspiration of the mucus secretions did not improve the atelectasis on the following CXR. Despite the lack of radiographic improvement, the patient passed a spontaneous breathing trial (SBT) and was successfully extubated three days later to humidified high flow nasal cannula (HHFNC).
A second therapeutic bronchoscopy was done seven days later and showed re-accumulation of thick mucoid secretions in left lung bronchi. Secretions were cleaned out again without radiographic improvement.
A third therapeutic bronchoscopy was done two days later and showed thick mucoid secretions extending from the proximal left main bronchus to the left upper lobe (LUL) and LLL bronchi. Therapeutic aspiration followed by lobar lavage using one liter of normal saline on room air temperature was performed without significant resolution of the atelectasis. Four days later, a fourth therapeutic bronchoscopy with washout of mucus plugs continued to have minimal radiographic improvement (Figure 4). The patient had completed a course of antibiotics for obstructive pneumonia at this point.
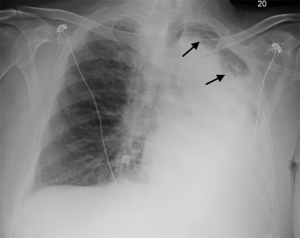
After two weeks of extensive management, a fifth therapeutic bronchoscopy continued to show mucoid secretions. The secretions were aspirated and two doses of 2.5 mg of rhDNase diluted in 7.5 mL of normal saline was instilled into the left upper and lower lobes. On the same day, a portable CXR performed a few hours later showed dramatic improvement in aeration of the LUL (Figure 5).
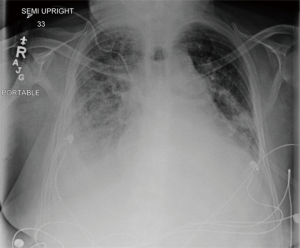
The procedure was repeated five days later for persistent atelectasis of the LLL. Two point five mg of dornase alfa was instilled in the basilar segments of the LLL with further clinical and radiological improvement (Figure 6).
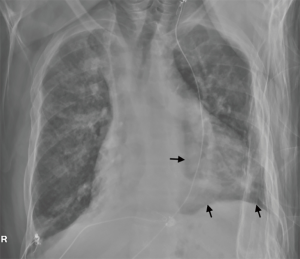
Ten days later, while the patient was on the medical floor, he developed hypoxemia with a pulse oxygen saturation (SpO2) of 67%. Stat CXR showed complete right lung atelectasis which was attributed to mucous plugging and possibly to the RML endobronchial tumorous lesion. A detailed discussion was held with the patient and his family where they opted for comfort measures. The patient expired peacefully a few days later.
Discussion
Lobar atelectasis occurs in a variety of medical conditions, such as critical illness, post-operative complication, trauma, and lung cancer. The main etiology of lobar atelectasis is bronchial obstruction by mucus plugs from buildup of copious purulent secretions due to defective clearance mechanisms. The management of atelectasis is usually conservative with aggressive pulmonary hygiene. Therapeutic bronchoscopy is performed in atelectasis unresponsive to conservative management.
Several case reports and small studies of bronchoscopically instilled rhDNase have reported promising outcomes in the pediatric population. In a study of 22 newborns with persistent atelectasis, nebulized rhDNase demonstrated significant radiographic and clinical improvement in 18 newborns after three days of treatment. The remaining four patients were considered to have refractory atelectasis, they improved on combined bronchoscopically instilled and nebulized rhDNase without serious side effects (4).
Slattery et al. published a case series of three children with cystic fibrosis (CF). They had refractory atelectasis after aggressive conservative management with chest physical therapy and aerosolized rhDNase. Bronchoscopic instillation of 2.5 mg of rhDNase in 10 mL of normal saline into the atelectatic lobe resulted in significant response in all three children the following day, with further improvement up to four weeks after DNase administration. No operative complications were reported (5).
In another case series, Hendriks et al. reviewed 30 non-CF pediatric patients (median age of 1.6 years) with persistent symptomatic atelectasis. Prior to treatment with rhDNase, sixteen patients were intubated, twenty-five were admitted to a pediatric intensive care unit, and five were treated in a medium care unit. Eighteen patients received nebulized rhDNase (2.5 mg twice daily) and twelve patients received droplet form rhDNase (0.25 mg diluted in 5 mL of 0.9% NaCl and given slowly as droplets through endotracheal tube or nasal cannula over 30 minutes, twice daily) (6).
Complete resolution of all atelectases within 24 hours was observed in three patients. Improvement on CXR was seen in 17 patients. No radiographic changes were seen in two patients. Clinical and radiographic deterioration occurred in three patients (all received endotracheal droplets and were mechanically ventilated). Two of the three patients had concurrent respiratory syncytial virus (RSV) bronchiolitis and one patient had congenital airway narrowing and adenovirus respiratory infection. Subgroup analysis of the three patients revealed no correlation between the deterioration and the viral infection. The deterioration was attributed to a rapid mobilization of the mucous.
Furthermore, a meta-analysis of 402 pediatric patients with non-CF pulmonary atelectasis (8 clinical trials and 12 case reports and series) also showed benefits in patients who received rhDNase for refractory atelectasis. Four of the studies attributed the atelectasis to mucous plugging and ineffective cough mechanisms, while other studies did not directly address the reason of the atelectasis. Doses of rhDNase varied between 0.2 mg daily to 2.5 mg every three hours. Administration was via nebulization or endotracheal droplets, and duration of treatment ranged from single dose to nine days. Six out of the eight clinical trials and ten out of the 12 case reports showed radiographic and/or clinical improvement, however timing of improvement ranged from few hours to 14 days after rhDNase administration (7).
Whitaker et al. showed that rhDNase was helpful in five adult and pediatric patients with CF who developed lobar atelectasis due to allergic bronchopulmonary aspergillosis (ABPA). A full recovery was eventually achieved by sequential bronchoscopy with local rhDNase instillation after failure of conservative treatment (8). Three of the patients had pseudomonas aeruginosa growth on sputum culture.
Similar outcomes were reported in another case series of five adult patients (age 20–26 years) with CF who developed lobar atelectasis regardless of the presence of ABPA. Recovery of the atelectasis was seen up to eight weeks after the rhDNase instillation (9).
The ability to extubate our patient despite persistent complete left lung collapse is likely due to the improvement of the bacterial pneumonia that, in turn, improved ventilation-perfusion (V/Q) mismatch and gas exchange. Although he had multiple failed therapeutic bronchoscopies, he showed significant radiographic and clinical improvement after bronchoscopic instillation of rhDNase. The success of the treatment is likely due to the ability of direct instillation to target mucous plugs in distal bronchi segments that could not be reached by bronchoscopy.
The safety profile of rhDNase has been demonstrated in studies of CF. The most common reported side effects are voice alteration (4–4.9%), skin rash (3.7–4.08%), laryngitis (1.02–1.3%), and less commonly, pharyngitis, fever, headache, chest pain, cough, and rhinitis (10-12). Most of these side effects were mild and no life-threatening complications were reported. Inhaled rhDNase has become part of the standard of therapy in the CF population.
Furthermore, refractory atelectasis can also occur in pregnancy. However, its safety has not been well established in this population. Reproductive studies have been performed in rats and rabbits at intravenous doses up to approximately 600 times the maximum recommended human dose (MRHD) in adults. No evidence of maternal toxicity, embryotoxicity, or teratogenicity was observed when rhDNase was administered throughout organogenesis (13).
In sum, despite there being no comparison to control groups in published literature, bronchoscopically instilled rhDNase seems to be safe and effective in adults with refractory lobar atelectasis who do not respond to conservative treatment. However, larger studies are needed before endorsing rhDNase as a standard of care.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patients for publication of this case report and any accompanying images.
References
- Bulbul Y, Eris B, Orem A, et al. Pulmonary atelectasis and survival in advanced non-small cell lung carcinoma. Ups J Med Sci 2010;115:176-80. [Crossref] [PubMed]
- Marini JJ. Acute Lobar Atelectasis. Chest 2019;155:1049-58. [Crossref] [PubMed]
- Puchelle E, de Bentzmann S, Zahm JM. Physical and functional properties of airway secretions in cystic fibrosis--therapeutic approaches. Respiration 1995;62 Suppl 1:2-12. [Crossref] [PubMed]
- Fedakar A, Aydogdu C, Fedakar A, et al. Safety of recombinant human deoxyribonuclease as a rescue treatment for persistent atelectasis in newborns. Ann Saudi Med 2012;32:131-6. [Crossref] [PubMed]
- Slattery DM, Waltz DA, Denham B, et al. Bronchoscopically administered recombinant human DNase for lobar atelectasis in cystic fibrosis. Pediatr Pulmonol 2001;31:383-8. [Crossref] [PubMed]
- Hendriks T, de Hoog M, Lequin MH, et al. DNase and atelectasis in non-cystic fibrosis pediatric patients. Crit Care 2005;9:R351-6. [Crossref] [PubMed]
- Thornby KA, Johnson A, Axtell S. Dornase Alfa for Non-Cystic Fibrosis Pediatric Pulmonary Atelectasis. Ann Pharmacother 2014;48:1040-9. [Crossref] [PubMed]
- Whitaker P, Brownlee K, Lee T, et al. Sequential bronchoscopy in the management of lobar atelectasis secondary to allergic bronchopulmonary aspergillosis. J Bronchology Interv Pulmonol 2011;18:57-60. [Crossref] [PubMed]
- McLaughlin AM, McGrath E, Barry R, et al. Treatment of lobar atelectasis with bronchoscopically administered recombinant human deoxyribonuclease in cystic fibrosis? Clin Respir J 2008;2:123-6. [Crossref] [PubMed]
- Aitken ML, Burke W, McDonald G, et al. Recombinant human DNase inhalation in normal subjects and patients with cystic fibrosis. A phase 1 study. JAMA 1992;267:1947-51. [Crossref] [PubMed]
- Fuchs HJ, Borowitz DS, Christiansen DH, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med 1994;331:637-42. [Crossref] [PubMed]
- Wagener JS, Rock MJ, McCubbin MM, et al. Aerosol delivery and safety of recombinant human deoxyribonuclease in young children with cystic fibrosis: a bronchoscopic study. Pulmozyme Pediatric Broncoscopy Study Group. J Pediatr 1998;133:486-91. [Crossref] [PubMed]
- Available online: https://www.drugs.com/pregnancy/dornase-alfa.html


