
Outcomes and cost-minimization analysis of cement spacers versus expandable cages for posterior-only reconstruction of metastatic spine corpectomies
Introduction
The vertebral column is the most common site of skeletal metastasis. Metastatic spine disease (MSD) commonly involves the vertebral body, and can cause structural instability of the spinal column and epidural compression. Patients can present with intractable pain, spinal instability, and debilitating neurological deficits (1). Treatment for MSD is typically palliative in nature, with the goal of improving quality of life through restoration or preservation of neurological function and easing of pain. Non-surgical interventions include radiation therapy and percutaneous vertebral augmentation; however, surgery may be necessary for epidural compression or spinal instability. Surgical management typically involves vertebral body resection with anterior column reconstruction and posterior fixation.
Surgical access for MSD is often invasive, a consideration not to be minimized in patients who frequently are quite sick. Anterior approaches via trans-thoracic or thoracoabdominal approaches have significant morbidity, risk to visceral organs and vasculature, and can be complicated by prior radiation therapy or surgery. Anterior approaches also require a separate procedure for posterior stabilization (1,2).
The posterior-only approach enables the surgeon to gain access to the posterior elements and perform direct spinal canal decompression, while still being able to work within the vertebral body for anterior and middle column decompression. Numerous studies have demonstrated that vertebral body reconstruction can be predictably and safely performed through a posterior-only lateral extracavitary approach (3-5).
Vertebral body reconstruction can be accomplished with either an expandable metal cage (EC) or a polymethylmethacrylate (PMMA) spacer. PMMA, specifically, is relative easy to use, inexpensive, and yields immediate stabilization. The cost of ECs is notably higher than the cost of PMMA. However, complications related to use of EC versus PMMA, and the longevity of each type of interbody device, are relatively unknown, particularly in the MSD patient population. Few studies have compared the relative successes between these two forms of reconstructions in the management of MSD. In this study, we compared both the outcomes and costs of EC and PMMA spacers in the treatment of MSD. We hypothesized that the rate of complications and revision surgery when using PMMA spacers to reconstruct the spine after corpectomy for MSD would be equivalent to use of an EC, with lower implant and operating room (OR) costs.
Methods
Sixty-five vertebral corpectomies for MSD required anterior column reconstruction from 2007–2014, and all were performed by the senior surgeon. Charts were retrospectively reviewed and no patients were excluded. All resections were performed via a posterior-only lateral extracavitary approach as single-stage procedures. Typically 3 cm of the medial rib were removed bilaterally at the affected level (if thoracic), which permitted sufficient visualization and access to the anterior vertebral body. If the rib was involved by the tumor, the affected portion was removed.
Twenty-nine patients underwent reconstruction with EC, and 36 patients received PMMA spacers. The decision for use of an EC or use of PMMA was made by the senior author; EC were utilized earlier in his practice, and he then switched to using almost exclusively PMMA spacers after initial good results were seen in patients. All EC implants were Stryker VLift cages (Kalamazoo, MI, USA). Simplex PMMA cement (Stryker, Kalamazoo, MI, USA) was used in all cement cases. Surgical technique followed published reports, and no lumbar nerve roots were sacrificed (3,5). Intraoperative imaging was used for both EC and PMMA placement, to insure correct placement and prevent inappropriate cement extravasation.
In PMMA reconstructions, one or two short Kirschner wires were inserted longitudinally into the vertebral bodies above and below the corpectomy to help reinforce the cement spacer and prevent it from migrating (Figure 1). In order to do so, the wire was advanced through the inferior endplate using a stout needle driver. The wire was then aligned more perpendicular to the endplate and advanced through the cephalad endplate. Cement was injected into the corpectomy defect using a simple 50 ml syringe, after allowing the cement viscosity to begin thickening so that the PMMA could be easily contained in the vertebral defect.
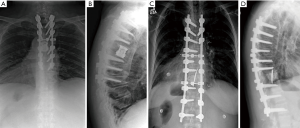
Preoperative radiographs, computerized tomography scans (CT), and magnetic resonance images (MRI) were assessed to determine tumor extent using Tomita staging (6). Spinal alignment preoperatively and postoperatively was assessed using coronal and sagittal Cobb angle measurements. Revised Tokuhashi scores were recorded to assess prognostic indicators, and the Charlson Comorbidity Index was used to quantify overall patient morbidity (7,8).
Postoperative radiographs were evaluated for implant failure or progressive malalignment. Other outcomes of interest included intraoperative time, perioperative complications, postoperative survival, and subsequent reoperations. Functional outcomes were assessed using Oswestry disability index (ODI) scores.
A cost minimization economic model was employed. Cost data were compiled using our institutional implant pricing, as well as recent published estimates for the cost of operative time in an orthopaedic OR setting (9). These OR costs were derived using time-driven activity-based costing (TDABC) methods, and were used as a proxy for our OR costs as our institution was not able to provide us this data. Two-way cost sensitivity analyses were then performed.
Statistical analysis included Student’s t-test for continuous variables, z-tests for proportions, and a log rank test for patient survival analysis. GraphPad (San Diego, CA, USA) statistical software was utilized.
Results
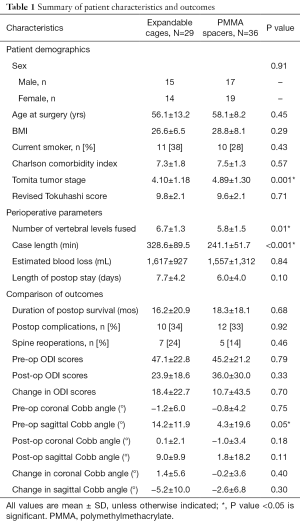
There were no preoperative baseline differences in gender, age at surgery, body mass index, smoking status, Charlson Comorbidity Index, or revised Tokuhashi score between the two treatment groups. The PMMA group did have a slightly higher mean preoperative Tomita stage, indicating more extensive spine involvement by tumor (4.89±1.30, 4.10±1.18, P=0.001) (Table 1).
Full table
Patients reconstructed with PMMA had a shorter mean operative duration by 87 minutes (328.6 vs. 241.1 minutes, P<0.001). No differences in estimated blood loss (EBL), hospital length of stay, or intraoperative/perioperative complications were found. Complications occurred in 34% of the patients in the EC group and 33% in the PMMA group (P=0.92) (Table 1). The most common complications in both groups were wound infections requiring incisional debridement (Table 2).

Full table
Postoperatively, there were no differences in the need for subsequent spinal reoperation (24% in EC patient and 14% in PMMA patients, P=0.46) (Table 1). The 7 reoperations in the EC cohort included one cage revision for an endplate fracture and implant subsidence that occurred 7 days after the index operation. This patient developed an infection after the revision and subsequently required an incisional debridement as well. Four patients required further decompression for recurrent tumor later in their disease course, and two additional patients required incisional debridement.
In the PMMA cohort, no patients required revision surgery for cement spacer failure or extrusion. Two patients required further decompression, one in the first week after the index operation. This patient also required incisional debridement after the second decompression procedure. One other patient returned for instrumentation extension and further decompression two years after the index operation, and two other patients required incisional debridement. No complications with cement displacement, Kirschner wire migration, or thermal injury to neural structures were noted.
There was no difference in patient survival between the cohorts (16.2±20.9 months for EC, 18.3±18.1 months for PMMA, P=0.68) (Figure 2). When analyzing radiographic outcomes, the EC patients had a slightly larger mean pre-operative sagittal Cobb angle (14.2° vs. 4.3°, P=0.05), but the postoperative Cobb angle and the overall changes in sagittal alignment were not different (Table 1). No differences were seen in the preoperative or postoperative ODI values between groups, nor when the changes in ODI scores were compared between the cohorts (Table 1).

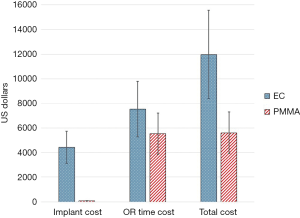
Because there were no significant differences in length of stay or blood loss, we assumed that hospital boarding costs and blood transfusion costs were similar between the cohorts. We identified implant costs and operative duration as the two primary cost variables between the EC and PMMA cohorts. Our institutional cost for an EC was $4,430, while one bag of PMMA was $75. Thus, implant cost savings were $4,355 favoring the PMMA cohort. PMMA patients had a mean surgical duration of 87 minutes less than EC patients. Using a literature-based OR time cost of $23/min, mean OR time savings were $2,001 for the PMMA cohort (9). Total cost minimization per PMMA case was thus $6,356, which was robust in 2-way sensitivity analyses varying both implant costs and time costs by 30% (Figure 3).
Discussion
Posterior-only approaches have been previously demonstrated to allow for circumferential spinal decompression and reconstruction through a single approach, with lower complication rates and less cost than combined anterior-posterior approaches (2-5). We have sought to quantify the longevity, complications, and relative costs of using EC and PMMA spacers to achieve stability after metastatic tumor corpectomy. This study is the largest series of posterior-only corpectomies for MSD with reconstruction using PMMA, to the knowledge of the authors. In addition, this is the first formal cost analysis comparing these two types of corpectomy reconstructions.
While the indications for corpectomies in metastatic disease patients are mainly palliative in nature, these patients are now living longer after cancer diagnosis, leading to an increasing incidence of MSD. In addition, patients are living with metastatic disease for longer periods of time, placing more biomechanical demands on their spinal constructs (10). How to treat those MSD patients who need surgery with the quickest, safest, and most cost-effective techniques is thus an important question for the health care system.
In our study, the effectiveness of treatment with either EC or PMMA was essentially equivalent between the treatments. There was no difference in postoperative survival between the two treatment groups. There were no disparities in radiographic alignment correction, complication rates, or reoperation rates. Operative time in the PMMA group was shown to be shorter compared to the EC group. The senior author attributed this to the relative ease of injecting PMMA into the corpectomy defect, whereas maneuvering an EC into place can take additional time to size the implant, as well as to resect additional bone to allow maneuvering of the cage into place while retracting and protecting neural structures.
Thus, our use of the cost minimization model was valid, as our results indicated that the effectiveness of treatment with either EC or PMMA were equivalent between treatments. Cost minimization analysis is only valid when this condition is met; otherwise, cost-effectiveness or cost-utility analyses must be performed (11,12). There are of course institutional differences in the contracted cost of EC implants, and in the cost of OR time. Our sensitivity analyses still demonstrated cost superiority of PMMA when varying these costs substantially. In aggregate, if all 65 cases had been performed with EC reconstruction, a total implant cost of $287,950 would have been incurred, versus just $4,875 with the use of PMMA for all cases.
This study focused on patients with metastatic disease. In reconstructions after resection of primary bone tumors of the spine, the authors do advocate using an EC as these patients are expected to have disease-free survival. In addition, if a patient presented with severe kyphosis from a metastatic lesion, using an EC can help to restore alignment (13). These factors would favor selection of the more expensive EC implant.
PMMA hardens via an exothermic process, so care must be taken in the spine to not place the cement in contact with the neural elements. In this series, we did not note any complications from nerve root or spinal cord damage due to heat injury. Our use of Kirschner wires placed into the vertebrae above and below the corpectomy was a simple way to help prevent dislodgement of the intercalary cement block.
Eleraky et al. reported a series of 32 posterior-only tumor resections for MSD, 16 of which were reconstructed with ECs and 16 with PMMA. Similar to our series, no differences in complications, stability, or reoperations were noted. These authors noted a trend towards better reduction of kyphosis in the EC patients, by approximately 5 degrees (14). Rajpal et al. reported on 37 thoracic and lumbar MSD corpectomies, 5 of which were reconstructed with PMMA. No PMMA patients and only 1 metallic cage patient required revision surgery (15).
Limitations of this study include its single-center design. Costs do likely vary between institutions and between regions of the country. Moreover, cancer patients are a heterogeneous population, and often many health expenditures result from other aspects of their illness than just MSD. Assignment of patients to each treatment cohort was not randomized, and was at the discretion of the senior surgeon. More of the EC cases were done earlier in the senior surgeon’s experience, likely biasing the surgical duration of the EC cases towards longer times. This expected learning curve was included in our rationale for the sensitivity analysis of the time cost, however. Despite this selection bias, we do posit that there are actual time savings using PMMA versus EC, given its ease of placement in uncured form.
Additionally, costs will vary somewhat between hospitals, based on vendor contracts. If a dedicated vertebroplasty-type cement kit with an injector is used, rather than the simple syringe we utilized, cement costs would also be expected to increase relative to the EC cost. Finally, we acknowledge that postoperative life expectancy and quality of life for patients with MSD likely improved over the course of this study. More active patients who are living longer would indeed place more stress on the reconstruction, and yet we did not find that the PMMA patients were experiencing failures or dislodgements.
In conclusion, the use of PMMA spacers is substantially less expensive in patients with MSD than use of ECs, while demonstrating equivalence in stability, spinal alignment, and risk of implant-related complications.
Acknowledgments
None.
Footnote
Conflicts of Interest: JM Buchowski, MD, MS receives royalty payments from Globus Medical and K2M. He receives institutional fellowship fudning from OMeGA and AOSpine North America. The other authors have no conflicts of interest to declare.
Ethical Statement: The data presented in this study was obtained following IRB approval from Washington University School of Medicine under a waiver of consent.
References
- Rose PS, Buchowski JM. Metastatic disease in the thoracic and lumbar spine: evaluation and management. J Am Acad Orthop Surg 2011;19:37-48. [Crossref] [PubMed]
- Archavlis E, Papadopoulos N, Ulrich P. Corpectomy in destructive thoracolumbar spine disease: cost-effectiveness of 3 different techniques and implications for cost reduction of delivered care. Spine 2015;40:E433-8. [Crossref] [PubMed]
- Shen FH, Marks I, Shaffrey C, et al. The use of an expandable cage for corpectomy reconstruction of vertebral body tumors through a posterior extracavitary approach: a multicenter consecutive case series of prospectively followed patients. Spine J 2008;8:329-39. [Crossref] [PubMed]
- Morales Alba NA. Posterior placement of an expandable cage for lumbar vertebral body replacement in oncologic surgery by posterior simple approach: technical note. Spine 2008;33:E901-5. [Crossref] [PubMed]
- Jandial R, Kelly B, Chen MY. Posterior-only approach for lumbar vertebral column resection and expandable cage reconstruction for spinal metastases. J Neurosurg Spine 2013;19:27-33. [Crossref] [PubMed]
- Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine 2001;26:298-306. [Crossref] [PubMed]
- Tokuhashi Y, Matsuzaki H, Oda H, et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine 2005;30:2186-91. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Hamid KS, Matson AP, Nwachukwu BU, et al. Determining the cost-savings threshold and alignment accuracy of patient-specific instrumentation in total ankle replacements. Foot Ankle Int 2017;38:49-57. [Crossref] [PubMed]
- Mariotto AB, Etzioni R, Hurlbert M, et al. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol Biomarkers Prev 2017;26:809-15. [Crossref] [PubMed]
- Rai M, Goyal R. Pharmacoeconomics in Healthcare. In: Vohora D, Singh G. editors. Pharmaceutical Medicine and Translational Clinical Research. Academic Press, 2017:465-72.
- Zhang S, Vora M, Harris AHS, et al. Cost-minimization analysis of open and endoscopic carpal tunnel release. J Bone Joint Surg Am 2016;98:1970-7. [Crossref] [PubMed]
- Sciubba DM, Gallia GL, McGirt MJ, et al. Thoracic kyphotic deformity reduction with a distractible titanium cage via an entirely posterior approach. Neurosurgery 2007;60:223-30. [PubMed]
- Eleraky M, Papanastassiou I, Tran ND, et al. Comparison of polymethylmethacrylate versus expandable cage in anterior vertebral column reconstruction after posterior extracavitary corpectomy in lumbar and thoraco-lumbar metastatic spine tumors. Eur Spine J 2011;20:1363-70. [Crossref] [PubMed]
- Rajpal S, Hwang R, Mroz T, et al. Comparing vertebral body reconstruction implants for the treatment of thoracic and lumbar metastatic spinal tumors: a consecutive case series of 37 patients. J Spinal Disord Tech 2012;25:85-91. [Crossref] [PubMed]


