
Clinical and prognostic significance of combined plasma fibrinogen concentrations and the monocyte-to-lymphocyte ratio in patients with ovarian cancer
Introduction
Ovarian cancer is ranked as one of the most prevalent lethal gynecologic malignancies (1). In the past several decades, the ability to recognize patients with early ovarian cancer has gradually improved because of the combined use of cytological analysis, an ultrasonic examination, an endoscopic approach, and other techniques. Therefore, the prognosis of these patients has also improved to some extent, and patients with early-stage ovarian cancer have a 5-year survival of 70–90% (2). However, early-stage ovarian cancer is usually asymptomatic, and the symptoms of late-stage disease are nonspecific (3). Therefore, more than 70% of these women are diagnosed as having stage III or IV disease, which has a 5-year survival rate of approximately only 25% (4). Moreover, some patients with early ovarian cancer develop recurrent disease or cancer-related complications. The main causes of recurrent disease are challenging to predict in management of early ovarian cancer. Therefore, early screening and assessing disease progression of patients with ovarian cancer are important, especially in the early stage. This is helpful for guiding individual anti-cancer treatment to improve the prognosis of patients. For the purpose of prognostic assessment, peripheral blood parameters of patients probably play an important role.
To date, the only clinically used biomarker for epithelial ovarian cancer (EOC) is carbohydrate antigen (CA) 125. However, CA125 has not been approved for use in diagnosis because it may be elevated in benign gynecological conditions, such as endometriosis. Furthermore, CA125 has a relatively low sensitivity of 50–62% for early-stage disease (5). Therefore, there are some clinical limitations for CA125 in discriminating between subclinical patients. Consequently, examining some more powerful prognostic markers of ovarian cancer than CA125 could be important.
Fibrinogen is mainly synthesized by hepatocytes, and it is an acute-phase reactant glycoprotein (6). Fibrinogen is important in regulating clot formation, wound healing, and the inflammatory response (7). Fibrinogen is not a traditional tumor biomarker. However, previous studies have shown that hyperfibrinogenemia is associated with an advanced International Federation of Gynecology and Obstetrics stage, more extensive residual disease, and poorer prognoses for patients with malignant diseases (8,9). Other studies have also indicated that elevated plasma fibrinogen concentrations are associated with prognosis in patients with ovarian cancer (10,11).
Systemic inflammatory responses play an important role in prognosis for a variety of cancers (12). The monocyte-to-lymphocyte ratio (MLR), which is a predictor of the inflammatory status, similar to the neutrophil-to-lymphocyte ratio (NLR), is a much more effective prognostic predictor in many solid tumors (3). Inflammation is a component of the tumor microenvironment and represents the seventh hallmark of cancer, and chronic inflammation plays a critical role in tumorigenesis (13). Several inflammatory response-related biomarkers in peripheral blood, such as the NLR, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio, have been widely investigated as potentially useful prognostic markers in different cancers (3). Xiang et al. (3) reported that the MLR may be clinically reliable and useful for accurately predicting initiation of ovarian cancer and subsequently for the patient’s prognosis. Furthermore, Marchetti et al. (14) reported that combining the NLR and fibrinogen concentrations could be used as a factor for predicting prognosis and response to treatment in patients who are affected by ovarian cancer.
In this study, we aimed to examine plasma fibrinogen concentrations and the MLR in patients with EOC. We further aimed to assess the clinical and prognostic significance of combined plasma fibrinogen concentrations and the MLR for patients with ovarian cancer.
Methods
Patient population
A total of 155 patients with EOC were reviewed in this retrospective study. The patients were from Hangzhou Cancer Hospital and Taizhou Central Hospital, and were hospitalized between May 2012 and March 2013. The selection criteria for patients were as follows: (I) EOC confirmed by pathology; (II) complete clinical, laboratory, imaging, and follow-up data; (III) no preoperative treatment, such as radiotherapy or neoadjuvant chemotherapy; (IV) no primary liver and kidney dysfunction; (V) no hypertension; (VI) no cardiovascular and cerebrovascular diseases; (VII) no other malignancies; (VIII) no acute inflammation and infections; (IX) no other diseases potentially activating the blood coagulation system; and (X) no autoimmune diseases or treatment with steroids. All of the patients were Han Chinese and diagnosed according to the histological diagnosis criteria. The stage of disease and histological types were in accordance with the International Federation of Gynecology and Obstetrics classification (15). All of the patients with serous EOC (except those with stage IA and IB disease) received six cycles of adjuvant carboplatinum and paclitaxel. Progression of cancer was diagnosed in the presence of elevated CA125 concentrations and imaging results according to the Response Evaluation Criteria in Solid Tumors criteria (16). Assessment of patients before treatment included clinical and biological data of patients during hospitalization, and the incidence of deep venous thrombosis and lymphatic or venous metastasis at the time of diagnosis. The survival time of follow-up included in this study was not longer than 5 years. Finally, after excluding patients with accidental death, we calculated the 5-year survival rate. In this study, the median follow-up time was 89.5 weeks (range, 5–261 weeks). At the same time, 102 patients with benign gynecological diseases who were matched for age, sex, and race, as well as matched to the above-mentioned relative selection criteria, were included in the analysis.
Laboratory assays
Blood samples were collected in sodium citrate- or EDTA-K2-containing tubes and anticoagulant-free tubes after an overnight fast on the morning before treatment. Plasma and serum were separated immediately and analyzed according to relative analytical instructions. For plasma fibrinogen measurement, an automatic coagulation analyzer (CS-5100; Sysmex Inc., Japan) and commercially available Thrombin reagents (Siemens Healthcare Diagnostics Products GmbH) were used. White blood cells (WBCs), including monocytes, neutrophils, and lymphocytes, were measured by an automatic hematological analyzer and commercially available reagents (BC-6900; Mindray Inc., China). The MLR and NLR were then calculated. Serum CA125, CA153, and CA199 concentrations were determined by a chemiluminescent analyzer (I2000; Abbott Co., USA).
Statistical analysis
The Student’s t-test and the Chi-square test were used for two samples of continuous and categorical variables for comparison of patients with EOC and controls, respectively. Receiver operating characteristic (ROC) curves were constructed for fibrinogen concentrations, the MLR, and the combination of plasma fibrinogen concentrations and the MLR (FC-MLR) as prognostic factors for EOC by plotting sensitivity versus 1-specificity. The area under the curve (AUC) was calculated for all indicators. The overall survival curves for mortality of patients with EOC were created by Kaplan-Meier analysis according to the cutoff values. Multivariate survival analysis was performed using the Cox proportional hazards regression model. The log-rank test was used to compare the differences in survival between the groups. All statistical analyses were performed with SPSS software (version 17.0). A P value of <0.05 was considered statistically significant.
Results
The present study initially investigated the basic characteristics of patients with EOC. Overall, approximately 60% of the patients were younger than 60 years, and the incidence of 5-year survival was 62.6%. At the time of diagnosis, 58.1% of patients had stage III or worse EOC. The incidence of deep venous thrombosis and lymphatic and venous metastases was 21.9%, 20.0%, and 14.2%, respectively, which was significantly lower than that of patients without deep venous thrombosis or metastasis (all P<0.01) (Table 1). Furthermore, we assessed the differences in biological and clinical parameters between controls and patients with EOC. We found that there was no significant difference in age between the two groups. Concentrations of fibrinogen, CA125, CA153, and CA199, and the MLR and NLR were significantly higher in patients with EOC compared with controls (P<0.05 for CA199, and P<0.001 for the other variables) (Table 2).
Full table

Full table
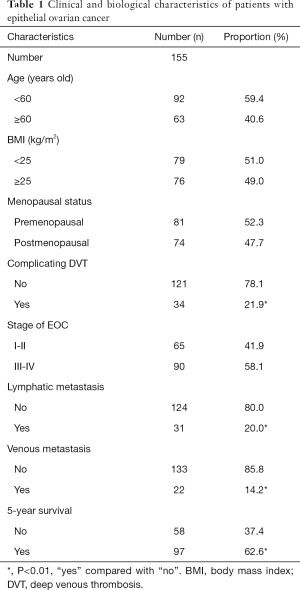
In this study, the associations of plasma fibrinogen concentrations with clinical characteristics of patients with EOC were examined. The mean plasma fibrinogen concentrations were 2.92±0.75 and 3.66±1.05 g/L in stages I–II and III–IV, respectively. Fibrinogen concentrations were significantly associated with the EOC stage (P<0.001, Figure 1A). Furthermore, fibrinogen concentrations were significantly higher in patients with lymphatic and venous metastasis than in patients without lymphatic and venous metastasis (4.03±1.00 vs. 3.18±0.92 and 4.86±0.88 vs. 3.10±0.92 g/L, respectively, both P<0.001; Figure 1B,C). Moreover, plasma fibrinogen concentrations were significantly lower in patients with 5-year survival than in patients without 5-year survival (P<0.001, Figure 1D). ROC curve analysis indicated a high AUC [0.755; 95% confidence interval (CI): 0.705–0.805; P<0.01] of plasma fibrinogen concentrations, with a cutoff value of 3.01 g/L for discriminating between patients with advanced and early EOC. Based on the cutoff value, the sensitivity and specificity were 0.78 and 0.65, respectively (Figure 1E). For the next study, patients were assigned to normal and high fibrinogen groups according to the cutoff value.
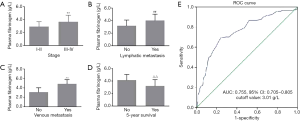
We further investigated the association of the MLR with clinical characteristics of patients with EOC. In patients with stages III–IV, the MLR was significantly higher than that in patients with stages I–II (0.27±0.06 vs. 0.22±0.07, P<0.001) (Figure 2A). The MLR in patients with lymphatic and venous metastasis was significantly higher than that in patients without lymphatic and venous metastasis (0.29±0.06 vs. 0.24±0.08 and 0.37±0.10 vs. 0.23±0.08, respectively, both P<0.001; Figure 2B,C). Furthermore, the MLR was significantly lower in patients with 5-year survival than in patients without 5-year survival (0.31±0.09 vs. 0.24±0.08, P<0.001; Figure 2D). ROC curve analysis showed an AUC of 0.774 (95% CI: 0.701–0.846, P<0.01) based on the cutoff value (0.23) of the MLR for discriminating between patients with advanced and early EOC. We also found that the sensitivity and specificity of the MLR were 0.82 and 0.62, respectively (Figure 2E). Subsequently, patients were divided into two groups including a normal and high MLR according to its cutoff value for the following study.
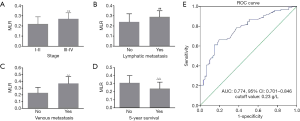
To assess the association of plasma fibrinogen concentrations combined with the MLR and progression of EOC, a ROC curve for discriminating between patients with advanced and early EOC was constructed. We found that FC-MLR had an AUC of 0.831 (95% CI: 0.767–0.895), and the sensitivity and specificity were 0.86 and 0.62 based on the cutoff values of fibrinogen and the MLR, respectively (Figure 3).
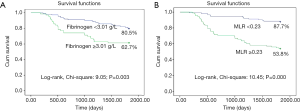
Furthermore, the 5-year survival rate was significantly lower in patients with high fibrinogen concentrations (≥3.0 g/L) or a high MLR (≥0.23) than in patients with low fibrinogen concentrations or a low MLR (62.7% vs. 80.5% or 53.8% vs. 87.7%, respectively, both P<0.01; Figure 4A,B). Fibrinogen concentrations and the MLR were associated with the prognosis for EOC.
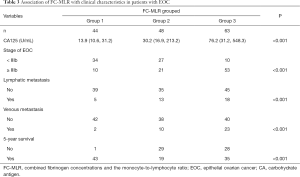
We further investigated whether there were differences in clinical characteristics between the groups based on the cutoff values of fibrinogen concentrations and the MLR (FC-MLR grouping). The patients were divided into three groups (group 1: no abnormality; group 2: either high fibrinogen concentrations or a high MLR; group 3: both high fibrinogen concentrations and a high MLR). We found that FC-MLR was significantly associated with CA125 concentrations, tumor stage, lymphatic metastasis, venous metastasis, and the 5-year survival rate (all P<0.001; Table 3).
Full table
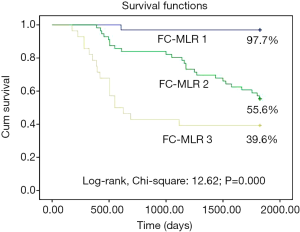
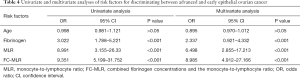
The 5-year survival rates of patients with EOC were 97.7%, 55.6%, and 39.6% in FC-MLR groups 1, 2, and 3, respectively, with a significant difference among the three groups (P<0.001; Figure 5). Univariate regression analysis showed that fibrinogen concentrations, the MLR, and FC-MLR were associated with the prognosis of patients with EOC (all P<0.001; Table 4). Multivariate regression analysis showed that fibrinogen concentrations, the MLR, and FC-MLR were also independent prognostic factors of EOC (all P<0.001; Table 4).
Full table
Discussion
The present study measured plasma fibrinogen concentrations and the MLR in 155 patients with EOC. We also assessed the association of combined FC-MLR with several clinical characteristics, including prognosis. One of the major findings in our study was that FC-MLR was significantly associated with the overall survival rate of patients with EOC, and patients with both high plasma fibrinogen concentrations and a high MLR had a lower overall survival rate.
Some previous studies have reported that elevated concentrations of plasma fibrinogen, which is an acute reactive protein, are characteristic of patients with cancer (8-10). Other studies have also indicated that hyperfibrinogenemia is associated with tumor progression and metastasis (17,18). However, the mechanisms of this process remain unknown. Yamaguchi et al. reported that interleukin-6, which is produced by cancer cells, can stimulate fibrinogen secretion in patients with lung cancer (19). Furthermore, Sahni et al. (20,21) showed that cancer cells also synthesize fibrinogen, and fibrinogen eventually promotes tumor cell growth and angiogenesis through interaction with fibroblast growth factor-2 and vascular endothelial growth factor. The present study showed higher plasma fibrinogen concentrations in patients with EOC than in those with benign gynecological disease. Furthermore, high fibrinogen concentrations were associated with the investigated clinical characteristics, including higher tumor stage, and lymphatic and venous metastasis. Therefore, our study indicated that increased fibrinogen concentrations were significantly associated with progression of EOC and disease conditions of patients. Moreover, based on the cutoff value of 3.01 g/L, fibrinogen had a high AUC and sensitivity in discriminating between early (stages I–II) and advanced (stages III–IV) tumors. Furthermore, we found that the 5-year survival rate was markedly lower in patients with EOC and higher fibrinogen concentrations. Therefore, our results suggest that fibrinogen measurement is important for predicting progression and prognosis of EOC.
A systemic inflammatory response causes variation in the balance of circulating WBC constituents (22). Lymphocytes possess a potent antitumor immune function that can inhibit progression of several tumors. Additionally, elevated lymphocytes are associated with a favorable prognosis of a variety of tumors. However, monocytes promote tumorigenesis and angiogenesis, and also inhibit the antitumor immune response in vivo (23). Therefore, there might a close association of increased monocytes and decreased lymphocytes with tumor progression. The MLR or lymphocyte-to-monocyte ratio could mirror the circulating immune status of the host, and this has been reported for a number of different malignancies (24). Therefore, the systemic inflammatory response can induce the occurrence of increased and decreased numbers of monocytes and lymphocytes during tumor progression. Lymphopenia is a surrogate marker of a weak immune response and an elevated monocyte count is a surrogate marker of a high tumor burden in the microenvironment. Therefore, patients with cancer and increased monocytes and decreased numbers of lymphocytes would probably show a decreased ability to inhibit progression of tumors, and the lymphocyte-to-monocyte ratio or MLR might be a good reflection of progression of cancer. Kwon et al. reported that the lymphocyte-to-monocyte ratio might be associated with treatment and survival outcomes in older patients with EOC (25). Furthermore, the lymphocyte-to-monocyte ratio is the most reliable independent prognostic factor of overall survival in patients with ovarian clear cell carcinoma (26). The present study also showed that patients with EOC had a higher MLR and other inflammatory parameters, such as the NLR, than those of patients with benign gynecological disease. Additionally, a high tumor stage, lymphatic metastasis, venous metastasis, and a low 5-year survival rate were associated with a high MLR. Therefore, our results suggest that patients with progressive EOC have a high MLR based on an increased number of monocytes and decreased number of lymphocytes because of an enhanced inflammatory response and reduced immune function. This could promote tumor angiogenesis and tumor growth, and also facilitate invasion and migration of tumor cells (23). Moreover, based on the cutoff value of 0.23, the MLR also had a high AUC and sensitivity in discriminating between early (stages I–II) and advanced (stages III–IV) tumors. This finding suggests that an elevated MLR is associated with progression of EOC, and it may also be used as a good progressive or prognostic parameter for patients with EOC.
The main finding of the present study was that the combination of plasma FC-MLR was a novel index for evaluating progression and prognosis of EOC. Regression analysis showed that fibrinogen concentrations, the MLR, and FC-MLR were independent risk factors of progression of EOC, but FC-MLR had high clinical significance in discriminating between early and advanced tumors. Furthermore, the ROC curve showed a greater predictive power than did fibrinogen concentrations or the MLR. To further assess the predictive significance of FC-MLR, patients with EOC were divided into three groups according to the cutoff values. We found that patients with both high fibrinogen concentrations and a high MLR had a high tumor stage, and high rates of lymphatic and venous metastasis than did those without high fibrinogen concentrations and a high MLR. We also found that patients with high fibrinogen concentrations and a high MLR were significantly associated with a lower 5-year survival rate, which is consistent with the above-mentioned results. Therefore, our study suggests that FC-MLR before treatment would be useful for assessing tumor progression and prognosis of patients with ovarian cancer. This information could be used to select patients who require neoadjuvant chemotherapy, which would be important for improvement of patients’ prognosis. FC-MLR is a simple index, which is obtained from fibrinogen concentrations and the calculated MLR derived from conventional blood analysis. Consequently, FC-MLR can be used as a cost-effective, convenient, practical, and powerful predictor for tumor progression and a prognostic index in patients with ovarian cancer.
There may be some limitations of the present study. In our study, we only reviewed 155 patients with EOC from two hospitals, and did not include patients with malignant ovarian germ cell tumors, which constitute approximate 25% of ovarian cancer. The results may have been affected by the small sample size, the fact that we performed a non-multicentric study, and the type of ovarian cancer. However, the present study showed notable significance of combined plasma fibrinogen concentrations and the MLR for assessing progression and prognosis of ovarian cancer. However, further controlled, prospective studies with a larger sample size, in multiple centers, and including all types of ovarian cancer may provide more definite results.
In conclusion, the present study suggests that combined plasma fibrinogen concentrations and FC-MLR is closely associated with tumor progression and prognosis in patients with ovarian cancer. FC-MLR may be useful predictor of tumor progression and prognosis for ovarian cancer.
Acknowledgments
The authors thank all those who participated in recruitment of patients and helping to gather patients’ information.
Funding: This work was supported by the Natural Science Foundation of Zhejiang Province (Grant No. LY17H080007).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of Zhejiang Provincial People’s Hospital. The study outcomes will not affect the future management of the patients. The use of human blood samples was in accordance with the legislation in China. Informed consent was obtained from the controls and patients or their relatives, and the study was approved by the institutional review board of the two hospitals involved in the study.
References
- Li H, Zhang W, Sun X, et al. Overexpression of kinesin family member 20A is associated with unfavorable clinical outcome and tumor progression in epithelial ovarian cancer. Cancer Manag Res 2018;10:3433-50. [Crossref] [PubMed]
- Kahramanoğlu İ, Tokgözoğlu N, Turan H, et al. YKL-40 in the diagnosis, prediction of prognosis, and platinum sensitivity in serous epithelial ovarian cancer. Turk J Obstet Gynecol 2018;15:177-81. [Crossref] [PubMed]
- Xiang J, Zhou L, Li X, et al. Preoperative monocyte-to-lymphocyte ratio in peripheral blood predicts stages, metastasis, and histological grades in patients with ovarian cancer. Transl Oncol 2017;10:33-9. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- McGrath SE, Annels N, Madhuri TK, et al. Engrailed-2 (EN2)-a novel biomarker in epithelial ovarian cancer. BMC Cancer 2018;18:943. [Crossref] [PubMed]
- Tennent GA, Brennan SO, Stangou AJ, et al. Human plasma fibrinogen is synthesized in the liver. Blood 2007;109:1971-4. [Crossref] [PubMed]
- Weisel JW. Fibrinogen and fibrin. Adv Protein Chem 2005;70:247-99. [Crossref] [PubMed]
- Feng Z, Wen H, Bi R, et al. Thrombocytosis and hyperfibrinogenemia are predictive factors of clinical outcomes in high-grade serous ovarian cancer patients. BMC Cancer 2016;16:43. [Crossref] [PubMed]
- Qiu J, Yu Y, Fu Y, et al. Preoperative plasma fibrinogen, platelet count and prognosis in epithelial ovarian cancer. J Obstet Gynaecol Res 2012;38:651-7. [Crossref] [PubMed]
- Luo Y, Kim HS, Kim M, et al. Elevated plasma fibrinogen levels and prognosis of epithelial ovarian cancer: a cohort study and meta-analysis. J Gynecol Oncol 2017;28:e36. [Crossref] [PubMed]
- Seebacher V, Aust S, D'Andrea D, et al. Development of a tool for prediction of ovarian cancer in patients with adnexal masses: Value of plasma fibrinogen. PLoS One 2017;12:e0182383. [Crossref] [PubMed]
- Mantovani A. The inflammation - cancer connection. FEBS J 2018;285:638-40. [Crossref] [PubMed]
- Orozco-Morales M, Soca-Chafre G, Barrios-Bernal P, et al. Interplay between cellular and molecular inflammatory mediators in lung cancer. Mediators Inflamm 2016;2016:3494608. [Crossref] [PubMed]
- Marchetti C, Romito A, Musella A, et al. Combined plasma fibrinogen and neutrophil lymphocyte ratio in ovarian cancer prognosis may play a role? Int J Gynecol Cancer 2018;28:939-44. [Crossref] [PubMed]
- Prat J. FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet 2014;124:1-5. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Zhang X, Long Q. Elevated serum plasma fibrinogen is associated with advanced tumor stage and poor survival in hepatocellular carcinoma patients. Medicine (Baltimore) 2017;96:e6694. [Crossref] [PubMed]
- Zhong H, Qian Y, Fang S, et al. Prognostic value of plasma fibrinogen in lung cancer patients: A Meta-Analysis. J Cancer 2018;9:3904-11. [Crossref] [PubMed]
- Yamaguchi T, Yamamoto Y, Yokota S, et al. Involvement of interleukin-6 in the elevation of plasma fibrinogen levels in lung cancer patients. Jpn J Clin Oncol 1998;28:740-4. [Crossref] [PubMed]
- Sahni A, Simpson-Haidaris PJ, Sahni SK, et al. Fibrinogen synthesized by cancer cells augments the proliferative effect of fibroblast growth factor-2 (FGF-2) J Thromb Haemost 2008;6:176-83. [Crossref] [PubMed]
- Sahni A, Francis CW. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood 2000;96:3772-8. [PubMed]
- Satomi A, Murakami S, Ishida K, et al. Significance of increased neutrophils in patients with advanced colorectal cancer. Acta Oncol 1995;34:69-73. [Crossref] [PubMed]
- Feng F, Zheng G, Wang Q, et al. Low lymphocyte count and high monocyte count predicts poor prognosis of gastric cancer. BMC Gastroenterol 2018;18:148. [Crossref] [PubMed]
- Ji H, Niu X, Yin L, et al. Ratio of immune response to tumor burden predicts survival via regulating functions of lymphocytes and monocytes in diffusel large B-cell lymphoma. Cell Physiol Biochem 2018;45:951-61. [Crossref] [PubMed]
- Kwon BS, Lee HJ, Yang J, et al. Prognostic value of preoperative lymphocyte-monocyte ratio in elderly patients with advanced epithelial ovarian cancer. Obstet Gynecol Sci 2017;60:558-64. [Crossref] [PubMed]
- Kwon BS, Jeong DH, Byun JM, et al. Prognostic value of preoperative lymphocyte-monocyte ratio in patients with ovarian clear cell carcinoma. J Cancer 2018;9:1127-34. [Crossref] [PubMed]


