
A 7.0–7.7% value for glycated haemoglobin is better than a <7% value as an appropriate target for patient-centered drug treatment of type 2 diabetes mellitus
Introduction
Diabetes mellitus is an increasingly important condition, the vast majority of cases being of the type 2 variety. More than four hundred million people lived with diabetes mellitus in the world in 2014, according to the World Health Organization (1). Diabetes mellitus is frequently associated with cardio-vascular disease, and the simultaneous presence of both conditions is associated with a significant mortality risk (2).
Glycated haemoglobin (HbA1c) is formed non-enzymatically (3), and has emerged as a major biomarker to evaluate patients with diabetes mellitus, since it serves as an index of longer term exposure to plasma glucose. Strengths and limitations of the use of HbA1c have been previously reviewed (4), and specific standards should apply to the employed assay method.
In a prospective investigation, glycated haemoglobin was shown to be associated with overall mortality in 4,662 men (5). In another study, glycated haemoglobin was associated with newly diagnosed diabetes and cardiovascular outcomes in 11,092 persons without diabetes or cardiovascular disease (data from the Atherosclerosis Risk in Communities study) (6).
Of particular importance is the evidence obtained in controlled clinical trials that have been carried out in the last decades. Early work in the University Group Diabetes Program (UGDP) study used fasting plasma glucose as the biomarker (7). However, later work established glycated haemoglobin as the main biomarker used in clinical trials.
The purpose of the present text is to help identity the optimal value for glycated haemoglobin to act as an appropriate target for patient-centered drug treatment of type 2 diabetes mellitus and to argue against the standards proposed by the American Diabetes Association. We have undertaken an analysis of this topic, taking into consideration the two following sets of trials:
- Major clinical trials in which lower values for glycated haemoglobin, under 7%, were reached in one or both arms of the study;
- Major clinical trials with a favourable overall impact on patients, represented by a decrease in overall mortality.
We further identified a third set of clinical trials not meeting any of the two criteria stated above, considered to be less relevant for the purpose of the present discussion.
This is not a formal review paper and therefore no search strategy was used. Instead widely known major clinical trials, namely those published in major medical journals, were used for the purpose of preparing the present text.
A major conclusion of the current text is that although HbA1c levels lower than 7% are seen in healthy populations without diabetes mellitus, lowering the HbA1c levels of persons with diabetes mellitus to values lower than 7% does not necessarily imply better overall outcomes for the patients.
A second conclusion is that no direct correlation has yet been established to exist between lowering plasma glucose and/or glycated haemoglobin and improving the overall outcomes of patients with diabetes mellitus.
A basic viewpoint is that empirical data obtained in intervention studies should be used to guide medical therapeutics, whenever such data exists, and not deductive reasoning based on epidemiological data (8).
A further viewpoint is that overall mortality represents basic prognosis of patients and should be the main topic to be evaluated when globally assessing therapeutics for diabetes mellitus.
Major clinical trials with glycated haemoglobin under 7%
In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, 10,251 patients with type 2 diabetes mellitus were under study, and were randomized to treatment either with intensive glucose control (5,128 patients) or standard glucose control (5,123 patients). The mean age was 62.2 years, 35% had had a previous cardiovascular event, and the median duration of diabetes was 10 years. After a mean follow-up of 3.5 years, the study was halted due to an increased mortality risk in the intensive treatment arm. The mean glycated haemoglobin values at the end of the study were 6.4% in the intensive therapy group and 7.5% in the standard therapy group (9). The primary outcome was a composite of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes, and occurred with a hazard ratio of 0.90; 95% confidence interval, 0.78 to 1.04.
Published in the same year, the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) study, included 11,140 patients with type 2 diabetes mellitus, divided between an intensive arm (5,571 patients) and a standard glucose control arm (5,569 patients) (10). The mean age of patients was 66 years and the mean duration of diabetes was 7.9–8 years. Patients under intensive therapy had a decreased incidence of nephropathy, but with no significant difference in overall mortality, myocardial infarction, stroke or retinopathy. The mean glycated haemoglobin values at the end of the study were 6.5% in the intensive therapy group and 7.3% in the standard therapy group. In a post-trial follow-up for a median of 5.4 years, no difference in overall mortality was found. However a decrease in end-stage renal disease was noted in the intensive therapy arm (11).
In the Veterans Affairs Diabetes Trial (VADT), 1,791 patients were randomized to intensive treatment versus standard glucose control (12). The mean age of the patients was 60.4 years, the mean duration of diabetes was 11.5 years and 40% of the patients had a previous cardiovascular event. At 6 months, a median glycated haemoglobin value was 6.9% in the intensive therapy group of patients, compared with 8.4% in the standard-therapy group. After a median follow-up period of 5.6 years, no significant differences were seen in the outcomes when both groups of patients were compared. Subgroup analysis of the trial, however, showed that intensive glucose lowering reduced cardiovascular events in those patients with less extensive calcified coronary atherosclerosis (13).
The Outcome Reduction with an Initial Glargine Intervention (ORIGIN) trial, involved “people 50 years of age or older with impaired fasting glucose, impaired glucose tolerance, or early type 2 diabetes in addition to other cardiovascular risk factors,” meaning that not all patients were persons with diabetes (14). Mean age was 63.6–63.5 years and mean duration of diabetes was 5.3–5.5 years. At seven years of treatment, a median glycated haemoglobin value of 6.2 was achieved in the insulin glargine group, whereas a value of 6.5 was the average in the standard care group (a value of 6.4 was noted in both groups at the beginning of the study) (14). Rates of incident cardiovascular outcomes were similar in the two groups of patients, and no significant difference was observed in overall mortality.
Major clinical trials with a favourable impact on overall mortality
The following clinical trials showed a favourable impact on overall prognosis (decreased mortality) of patients with diabetes mellitus.
In the United Kingdom Prospective Diabetes Study (UKPDS) 33 report, 3,867 newly diagnosed patients with type 2 diabetes were studied in an intensive treatment arm, compared to conventional policy. The former group had a 10-year median glycated haemoglobin value of 7.0% (6.6% at the initial period, 8.1% at the terminal phase of the trial) compared with a 10-year median glycated haemoglobin value of 7.9% in the control group. No significant reduction in either overall mortality or myocardial infarct were seen in the two groups of patients (15).
In the UKPDS 34 overweight study (involving 753 patients, with a mean age of 53 years), those in the metformin arm obtained a 10-year median value of 7.4% for glycated haemoglobin (6.7% at the initial part, 8.3% at the terminal phase of the study; 8.0% for the control group). A significant reduction of overall mortality (of 36%) and myocardial infarction were reported in the metformin-treated patients (16).
A 10-year post-trial monitoring follow-up study of 3,277 patients was carried out, and a 13% reduction in mortality was seen in the intensive therapy sulfonylurea–insulin treated group of patients, whereas a 27% reduction in mortality was reported for the metformin-treated group of patients (17). Glycated haemoglobin was above 8% for a significant part of the time in all of the studied groups (17).
In the Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes (EMPA-REG OUTCOME) study, more than 99% of patients had established cardiovascular disease (mean age 63.2–63 years). A marked reduction in overall mortality (32% relative risk reduction) and a reduction of a combined endpoint (death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke) were realized in the empagliflozin-treated patients (18). Furthermore, there was a 35% reduction in hospitalization for heart failure with empagliflozin. Mean glycated haemoglobin in the empagliflozin arm was 7.93% at the end of the study for patients treated with 10 mg empagliflozin and 7.81% for the 25 mg-treated patients, compared with 8.16% for placebo-treated patients.
A significant mortality benefit was not seen in the Canagliflozin cardiovascular assessment study (CANVAS), and so this trial will not be discussed at further length (an increased risk of amputation was noted) (19). Dapagliflozin also failed to show a survival benefit in the Dapagliflozin effect on cardiovascular events–thrombolysis in myocardial Infarction 58 (DECLARE–TIMI 58) trial, even though a lower rate of hospitalization for heart failure was seen with active treatment (20). Mean glycated haemoglobin in this latter trial was above 7.5% in both treatment arms.
In the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial, liraglutide, a glucagon-like peptide 1 (GLP-1) analogue, was compared with placebo (4,668 versus 4,672 patients) (21). The mean age was 64.2–64.4 years, the mean duration of diabetes was 12.8 years, 81.3% of patients had established cardiovascular disease and 72.4% had chronic kidney disease. After a median follow-up of 3.8 years, the liraglutide arm had a 15% significant reduction in overall mortality. Mean glycated haemoglobin was over 7.5% at the end of the study in liraglutide-treated patients (21). At 36 months, “a mean difference between the liraglutide group and the placebo group of −0.40 percentage points” was seen (21).
A reduction in overall mortality was also seen with exenatide (also a GLP-1 analogue) in the Exenatide Study of Cardiovascular Event Lowering (EXSCEL) (22). A total number of 14,752 patients were under study (the majority of whom with previous cardiovascular disease), with a median follow-up of 3.2 years. Although, in this study major adverse cardiovascular events were not significantly decreased by exenatide when compared to placebo, overall mortality (a secondary outcome) decreased, with a hazard ratio of 0.86 (and 95% confidence interval of 0.77−0.97). In EXSCEL, at baseline the median glycated hemoglobin value was 8.0% (22), and median values for glycated haemoglobin stayed clearly above 7.0% in both arms of the study.
A mortality benefit was not seen with semaglutide in the Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6) and so this trial will not be discussed at further length (23). The same happened with albiglutide in the Harmony-Outcomes trial. This was a long term, randomised, double blind, placebo-controlled study to determine the effect of albiglutide when added to standard blood glucose lowering therapies, on major cardiovascular events in patients with type 2 diabetes mellitus (24), in which mean glycated haemoglobin was above 7.5% in both arms.
Lack of overlap between the two sets of clinical trials
As presented above and in Table 1, the list of clinical trials with a mean/median glycated haemoglobin under 7% in at least one of the arms and the list of clinical trials with a favourable overall impact (mortality decrease) do not overlap. This evidence indicates that achieving levels under 7% are not shown to decrease mortality. Ethical issues have been raised concerning pharmacologically induced decrease of glycated haemoglobin in patients with diabetes to low values, in particular given the results of the ACCORD trial (25). Furthermore, reducing levels below 7% tends to increase the occurrence of hypoglycaemia (26). For example, ADVANCE reported nearly double the incidence of severe hypoglycaemia in the intensively treated arm (10). A serious economic impact also results from establishing low targets for glycated haemoglobin in these patients, arguably using limited resources that could perhaps be better used otherwise.
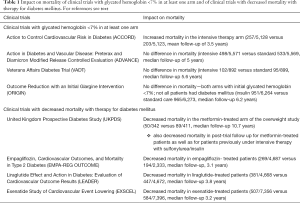
Full table
Evidence from a number of controlled clinical trials conducted in the last decades shows that glycated haemoglobin levels that are more appropriate for patients, in the sense of having fewer adverse clinical outcomes, namely death, do not correspond to values found in populations without diabetes. A limitation is that clinical trials carried out in patients with diabetes indicate rather different mortality rates, especially in relation to renal function (27). Other sources of evidence, apart from clinical trial data, may also be of interest, but with a lower level of applicability. Epidemiological data reported by Currie et al. shows a survival benefit for patients with type 2 diabetes mellitus with glycated haemoglobin in the vicinity of 7.5% (28). After breaking patients into 10 deciles, the study offers a graph of hazard ratios of mortality in comparison with the low point of around 7.5%. The result is a U shaped curve, with greater mortality both below and above 7.5% (28). Huang et al. reported a similarly U-shaped relationship between mortality and HbA1C values in patients with diabetes mellitus aged ≥60 years (29).
Major clinical trials neither with glycated haemoglobin under 7% nor with a favourable impact on overall mortality
A further set of clinical trials have not met any of the two criteria presented above (either glycated haemoglobin under 7% or a favourable impact on overall mortality). They are considered less relevant for the purpose of the present discussion, except as they strengthen the major viewpoints and premises of the present text, as stated above.
This set of trials includes, namely, major clinical trials using dipeptidyl peptidase-4 inhibitors (30-32), a class of drugs with no known favorable clinical effects in patients with diabetes mellitus. Saxagliptin was noted, in fact, to lead to an increase in hospitalizations for heart failure (31).
Degludec (when compared to glargine) was shown not to decrease mortality (33). Pioglitazone, in the Prospective pioglitazone clinical trial in macrovascular events, also failed to significantly decrease overall mortality, even if a decrease of 0.8% in glycated hemoglobin was seen with pioglitazone, from a baseline value of 7.8% (34).
Clinical trials carried out in the context of myocardial infarction/acute coronary syndrome are not discussed in the present text.
Critical evaluation of current guidelines on the issue under discussion
The evidence presented above supports HbA1c levels not lower than 7% as yielding, based on current knowledge, the best results when treating patients with type 2 diabetes. These findings reject an HbA1c of <7% and as well as target values >8% in the treatment of these patients.
The results from clinical trials are in contrast to the central conclusion of current American Diabetes Association recommendations: not only that “Goals should be individualized based on duration of diabetes, age/life expectancy, comorbid conditions, known CVD or advanced microvascular complications, hypoglycemia unawareness, and individual patient considerations” (26), but also that a reasonable A1C goal for many nonpregnant adults is <7% (53 mmol/mol)” (26). This position is presented in the American Diabetes Association and the European Association for the Study of Diabetes (ADA/EASD) joint position statement, suggesting a “patient- centered approach” (26,35).
Following an appropriate claim, supported by the studies we cite, that overaggressive treatment in some patients may not have beneficial results, these authors succinctly state their patient-centered perspective: “…instead of a one-size-fits-all approach, personalization is necessary, balancing the benefits of glycemic control with its potential risks, taking into account the adverse effects of glucose-lowering medications (particularly hypoglycemia), and the patient’s age and health status, among other concerns” (35). We take issue with their proposed version of a “patient-centered approach”.
The meaning of “patient-centered treatment” is not spelled out in the ADA/EASD proposal. It appears to mean that medical advice should depend on a matrix involving seven characteristics, all of which are to be evaluated for each patient: risk of hypoglycemia and adverse reactions, disease duration, life expectancy, comorbidities, vascular complications, patient motivation, and patient’s support system. This approach is reiterated in the ADA 2019 recommendations for Glycemic Targets (26). These seven factors come in a continuous variety of shades, as graphically represented in a colourful chart, with a ramp depicting the level of each trait (35). The target, written in the middle of the ramps, is what seems to be the norm HbA1c of 7%. This number is then accepted, lowered or raised depending on the joint values of all the traits: “Thus, characteristics/predicaments toward the left justify more stringent efforts to lower HbA1c, whereas those toward the right suggest (indeed, sometimes mandate) less stringent efforts” (35). Considering one trait as an example, a person with very low motivation, and so is unlikely to adhere to strict control, would have a target HbA1c higher than 7%, while a highly motivated person presumably has a target lower than 7%. Unfortunately for the scheme, traits indicating a high value may conflict with traits indicating a low value, e.g., a motivated patient, indicating a more stringent target, may have a weak support system, indicating a less stringent target. No way is suggested to prioritize or balance conflicts among traits.
This plan is patient-centered in that the traits involved provide basis for the physician to fine tune a target for each patient. While presumably a medical decision, it is a decision that is not, and probably cannot be, supported by the best available evidence in clinical trials. This is the case because this plan is computationally complex, undecidable nearly in principle. Even if most of these traits are specifiable, sometimes on a qualitative and sometimes a quantitative scale, the schema also involves traits that a physician or even a patient cannot be fully knowledgeable about, such as the patient’s motivation or susceptibility to side effects.
The plan involves three basic mistakes from the point of view of the best available evidence. First of all, the approach apparently proposes for many patients a target below 7%, perhaps significantly below. One of the few available examples of an abbreviated application of the system does just that: “[H]ealthier patients with long life expectancy accrue risk for vascular complications over time. Therefore, lower glycemic targets (e.g., an HbA1c,6.5–7.0%)… should be achieved.” (36). The ADA currently recommends under 7% for many non-pregnant patients and under 6.5 for selected patients (26). As we show in the present report, 7% considered as typical in the scheme, is at the low end of the appropriate interval. The ADA/EASD proposal recommends a more risky approach for patients at the “good” end of the traits. Second, the ADA/EASD approach indicates that the target for many patients is higher than 8%. Under the scheme, this target would be appropriate for patients at the “bad” end of the scales. Third, the plan’s approach is ad hoc, something like pulling a number out of a hat. There is no algorithm involved, just a potentially indefinite degree of highly likely conflicting sometimes qualitative and sometimes quantitative values.
It is certainly true that targets may need to be adjusted to patient characteristics. One could argue that an 80-year old person with diabetes suffering from late stage Alzheimer’s disease might not be chronically treated at all, on the basis that this type of patient has not been studied in clinical trials, namely in the clinical trials mentioned above. Some physicians might argue that acute metabolic derangements should not be treated; others might argue that would be excessive; in general, a case can be made against automatically applying the conclusions of clinical trials to populations of patients not studied in the trials—the problem of external validity of the conclusions of the trials (37).
The ADA/EASD plan is different from establishing a target with the understanding that a patient with special circumstances might require altering the target. In the ADA/EASD plan every patient is considered unique by his or her complex characteristics. Under these circumstances, informed consent is virtually impossible. In short, there is no way to know the benefits or the risks of the physician and/or patient selected target, at least for those patients falling outside the 7.0–7.7 target (now suggested; see below).
Informed consent by its nature is patient-centered. It requires that patients be informed about expected benefits and risks of proposed treatment. Also, the informed consent process may contain information about medically appropriate alternative treatments, should any other appropriate options exist. Informed consent conveys medical judgment. It does not substitute patient views for that judgment, except insofar as patient rejects treatment (38). The obligation of the physician is relay to the patient the best medical advice available to the physician. In mutual discussion, the decision to accept or reject that advice rests with the patient.
Clinical trials demonstrate that the target should not be under 7%. Physicians have a moral and often legal informed consent obligation, at least under typical circumstances, to present to the patient the best available treatment. Under the ADA/EASD plan, every patient becomes an ersatz clinical trial of one, but a trial that mainly influences the physician doing the “experimenting.” It is not genuinely experimental, because generalizable knowledge is not the goal (35). Informed consent under these circumstances would be flaccid, mere speculation. But given the risky quasi-experimental nature of the treatment, under the schema, a patient has a right to a rigorous informed consent (25).
An ideal and genuine patient-centered approach provides a proposed plan of treatment based on the best available medical advice, and offers that plan in an informed consent process that provides a clear explanation of the risks and expected benefits, also in relation to the best available evidence. A patient requires expert advice from his or her physician, which should be based on the best available evidence.
The ADA/ESAD guidelines involving seven factors are not supported by the evidence. In fact, they involve items that are not controllable and may induce bias. This is true about a patient’s resource and support system, where it is easy to believe that members of minority groups may lack appropriate resources. Since there is no evidence to support the combined use of seven factors, the physician who relies on them cannot offer expert opinion as a way to allow a patient to provide informed consent. The informed part depends on input from the physician, while consent comes from the patient; in consultation with the physician the patient applies his or her standards to the expected benefits and expect burdens of treatment as these are presented by the physician.
Suggested targets for therapy
What then would be the appropriate target for drug therapy of type 2 diabetes mellitus? To answer this question, we assume total mortality to be the major factor to be taken into consideration. The two clinical trials that have yielded the best results regarding overall mortality are UKPDS and EMPA-REG OUTCOME. We take data from the former trial to establish the lower end of the target range: 7.4 for UKPDS 34, metformin data; 7.0 for UKPDS 33. We next take data from the latter trial to establish the upper end of the target range. For that purpose, we have analysed the mean values for HbA1c for the EMPA-REG OUTCOME clinical trial. Considering sets of data of mean HbA1c (adjusted; including data from all patients, irrespective of taking the drug or not; or receiving rescue medication or not) for 206 weeks, a mean value of 7.67 was recorded in patients treated with 10 mg empagliflozin, 7.59% for the 25 mg-treated patients, and 8.02% for placebo-treated patients (B Zinman, personal communication).
We therefore submit a target level for HbA1c of 7.0% to 7.7% for drug treatment of type 2 diabetes mellitus. A more conservative approach would indicate 7.4–7.7%, since in UKPDS 33 an impact on mortality was not recorded, except in the post-trial follow-up study. These limits, based on clinical trials, alongside the risks and expected benefits, should be conveyed to the patient. These limits include the observed values both for UKPDS 33 and UKPDS 34 (metformin data—overweight study) and fall outside of the observed values for the control arm of UKPDS. The limits also include the 7.5 value seen in the observational data cited above. These limits should be revised if and when further clinical trials are published that surpass UKPDS and/or EMPA-REG OUTCOME relating to overall mortality.
Limitations of the present text
The present text is limited by the relatively heterogeneous sources of data, including age and risk profile of the populations of patients under study in each clinical trial.
In recent years, a considerable amount of data has been obtained involving obese patients who underwent bariatric surgery. It is well established that a large proportion of patients with diabetes show significant improvements in their glycaemic profile, or even a “cure” of diabetes mellitus (remission might be a better word) in association with considerable weight loss, as well as with arterial hypertension and sleep apnoea (39). The considerations we presented do not apply to patients under weight loss programs; instead they apply to patients under pharmacological treatment of diabetes mellitus, as studied in the cited clinical trials.
Regulatory consequences and general conclusions
In light of the evidence reviewed, regulatory authorities should not accept arguments in favour of considering “inadequately controlled” those patients with diabetes with a glycated haemoglobin not under 7%, since not one single major clinical trial has shown an improvement in overall prognosis when values under this threshold were reached. Therefore, no strategy indicating an under 7% target for glycated haemoglobin has a sound clinical trial basis, even if it is labelled “patient-centered”. On the other hand, drugs such as metformin, empagliflozin, liraglutide and exenatide have been shown to favourably improve prognosis of patients with type 2 diabetes, but not by reaching near normal glycated haemoglobin levels.
Glycated haemoglobin levels of 7% to 7.7% have yielded, up to the present moment, the best results when treating patients with type 2 diabetes.
Departure from these values could be justified under any the following circumstances (Table 2):
- A patient with clinical characteristics markedly different from the patients studied in the trials;
- A patient with drug intolerance preventing the attainment of the target presented above;
- A patient with a different preference regarding drug therapy;
- A patient under a weight loss program;
- An older patient with frailty, dementia, last year of life;
- A pregnant patient.

Full table
This summary statement presents what we consider a reasonable patient-centered therapy.
Acknowledgments
The authors thank Dr. Bernard Zinman for kindly supplying data from the EMPA-REG OUTCOME clinical trial. The publication charges were paid by Faculdade de Medicina da Universidade do Porto, under Programa Doutoral em Cuidados Paliativos.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- World Health Organization. (2016). Global report on diabetes. Available online: http://www.who.int/iris/handle/10665/204871
- Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229-34. [Crossref] [PubMed]
- Rahbar S. The discovery of glycated hemoglobin: a major event in the study of nonenzymatic chemistry in biological systems. Ann N Y Acad Sci 2005;1043:9-19. [Crossref] [PubMed]
- Lippi G, Targher G. Glycated hemoglobin (HbA1c): old dogmas, a new perspective? Clin Chem Lab Med 2010;48:609-14. [Crossref] [PubMed]
- Khaw KT, Wareham N, Luben R, et al. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of european prospective investigation of cancer and nutrition (EPIC-Norfolk). BMJ 2001;322:15-8. [Crossref] [PubMed]
- Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010;362:800-11. [Crossref] [PubMed]
- Meinert CL, Knatterud GL, Prout TE, et al. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. Mortality results. Diabetes 1970;19 Suppl:789-830. [PubMed]
- Nunes JPL. Medical therapeutics: from induction to scientific evolution. Perspect Biol Med 2013;56:568-83. [Crossref] [PubMed]
- Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545-59. [Crossref] [PubMed]
- Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560-72. [Crossref] [PubMed]
- Zoungas S, Chalmers J, Neal B, et al. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med 2014;371:1392-406. [Crossref] [PubMed]
- Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129-39. [Crossref] [PubMed]
- Reaven PD, Moritz TE, Schwenke DC, et al. Intensive glucose-lowering therapy reduces cardiovascular disease events in veterans affairs diabetes trial participants with lower calcified coronary atherosclerosis. Diabetes 2009;58:2642-8. [Crossref] [PubMed]
- Investigators OT, Gerstein HC, Bosch J, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319-28. [Crossref] [PubMed]
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837-53. [Crossref] [PubMed]
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:854-65. [Crossref] [PubMed]
- Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577-89. [Crossref] [PubMed]
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015;373:2117-28. [Crossref] [PubMed]
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 2017;377:644-57. [Crossref] [PubMed]
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2019;380:347-57. [Crossref] [PubMed]
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2016;375:311-22. [Crossref] [PubMed]
- Holman RR, Bethel MA, Mentz RJ, et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2017;377:1228-39. [Crossref] [PubMed]
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 2016;375:1834-44. [Crossref] [PubMed]
- Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018;392:1519-29. [Crossref] [PubMed]
- DeMarco JP, Ford PJ, Patton DJ, et al. Is there an ethical obligation to disclose controversial risk? A question from the ACCORD Trial. Am J Bioeth 2014;14:4-10. [Crossref] [PubMed]
- 6. Glycemic Targets: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019;42:S61-70. [Crossref] [PubMed]
- Barkoudah E, Skali H, Uno H, et al. Mortality rates in trials of subjects with type 2 diabetes. J Am Heart Assoc 2012;1:8-15. [Crossref] [PubMed]
- Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet 2010;375:481-9. [Crossref] [PubMed]
- Huang ES, Liu JY, Moffet HH, et al. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care 2011;34:1329-36. [Crossref] [PubMed]
- Green JB, Bethel MA, Armstrong PW, et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2015;373:232-42. [Crossref] [PubMed]
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus. N Engl J Med 2013;369:1317-26. [Crossref] [PubMed]
- Rosenstock J, Perkovic V, Johansen OE, et al. Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA 2019;321:69-79. [Crossref] [PubMed]
- Marso SP, McGuire DK, Zinman B, et al. Efficacy and Safety of Degludec versus Glargine in Type 2 Diabetes. N Engl J Med 2017;377:723-32. [Crossref] [PubMed]
- Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279-89. [Crossref] [PubMed]
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140-9. [Crossref] [PubMed]
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577-96. [Crossref] [PubMed]
- Rothwell PM. External validity of randomised controlled trials: “To whom do the results of this trial apply? Lancet 2005;365:82-93. [Crossref] [PubMed]
- Jones GE, DeMarco JP. Bioethics in Context: Moral, Legal, and Social Perspectives. Peterborough, Ontario, Canada, 2016.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724-37. [Crossref] [PubMed]


