
Non-small cell lung cancer 2 cm or less: robotic segmentectomy sets the gold standard against non-surgical therapy
Dr. Nguyen and colleagues presented a retrospective review of the long-term results of robotic anatomic segmentectomy for patients with clinically-staged T1a and T1bN0M0 non-small cell lung cancer (NSCLC) (1). This is a well-written paper with a timeline that spans from 2004–2013, allowing for a minimum follow-up of 5 years. It appears that follow-up is complete in all patients. If true this is an important feature that few studies (including all of ours) have been able to accomplish.
As shown in Table 1, the author’s surgical outcomes are not much different from our first report of 100 robotic segmentectomies (2) and our most recent experience on 245 robotic segmentectomies (3).
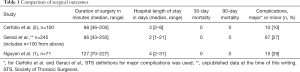
Full table
Given the similarities in the short-term outcomes, the next question to pose is: “Are the more important long-term outcomes the same?”. The methodology of postoperative patient follow-up is the key in these types of studies. It appears that no patients in this study were lost to follow-up (which is unusual but commendable) and all patients had postoperative surveillance CT scans at the desired frequency. The overall 5-year survival, however, is low at 43%. The cancer-specific 5-year survival is also low at 55%. These are quite different from our multi-institutional paper on 1,339 patients who underwent robotic lobectomy for lung cancer. The 5-year stage specific-survival in our series was: 83% for stage IA NSCLC, 77% for stage IB, 68% for stage IIA, 70% for stage IIB, 62% for stage IIIA and 31% for stage IIIB (4). In Dr. Nguyen’s series, pathologically-upstaged patients fared even worse, with an overall and cancer-specific 5-year survival of 0%. A reason for this offered by the authors may be due to the less accurate non-operative staging modalities used in the two studies that may cause pathologic upstaging in up to 30% of patients from baseline pretreatment stage. When adjusted for pathological stage I NSCLC, the overall 5-year actuarial survival was 55% and cancer-specific 5-year actuarial survival was 73%. These differences are important since we need to set an honest baseline for the gold standard for surgery in order to compare stereotactic radiosurgery and radiofrequency ablation. In addition, robotic bronchoscopy is already here and incisionless treatments for lung cancers that are less than 2 cm in size are on the immediate horizon. Further well-performed and longitudinal studies are needed to accurately answer these questions.
Technical comments
From a purely technical perspective we wish to review a few operative details. The authors suggest that the port placement and sequence of a robotic segmentectomy operation has not yet been standardized. We respectfully disagree as one standard is now taught in approved advanced robotic courses irrespective of the teacher. Any process no matter how complex or simple can find an optimal conduct. Highly functional teams should always be seeking new and better ways to perform it, but the current optimal conduct of an operation should be well-described. We previously have reported a standardized operative conduct for robotic pulmonary resection that we believe optimizes efficiency and quality (5). After pleural inspection, the appropriate regional N1 lymph node is removed first and sent for frozen pathologic review. During this wait-limiting part of the operation the 5 stations of the N2 lymph nodes are removed (#9, 8, 7, 4R and 2R in that order on the right side of the chest, and in the left chest we prefer #9, 8, 7, 5, 6 in that order). We have previously outlined selected robotic segmentectomy operations (6) and have recently attempted to identify and describe the optimal conduct for each and every possible robotic segmentectomy operation (segments 1–10 on the right side and segments 1–9 on the left) in an upcoming book chapter. Dr. Nguyen and colleagues state they traditionally prefer to pursue robotic lobectomy rather than robotic basilar segmentectomy in both the right and left chest, which is acceptable however this preference remains lesion-, patient-, and surgeon-dependent. The basilar segments of the lower lobe can undergo a formal segmentectomy and may provide value in selected patients over large wedge resection.
We do not favor lung inflation as the optimal way to identify the intersegmental plane. It adds time—requiring the anesthesiologists to manipulate the double lumen endotracheal tube (DLET) to inflate the lung, leading to loss of view and the operative domain, plus additional time waiting for the lung to re-deflate. Additionally, an inflation/deflation demarcation line can be misleading due to peripheral communication via interalveolar connections (the pores of Kohn). Furthermore, more time is required for the lung to deflate, which is not insignificant given many of these patient’s degree of emphysema. It is important to point out that inflating the divided bronchus after stapling and then cannulating it via a butterfly-type needle and injecting air to inflate the segment that is to be removed (not described in this paper but used by some) can add significant risk of air embolism (7,8). For this reason, as well as the fact it is cumbersome and adds time and cost, we do not recommend this technique of segment identification. We prefer indocyanine green (ICG) for several uses: transbronchial administration for nodule localization via a navigational bronchoscopy prior to double lumen tube placement, and intravenous administration for intersegmental plane identification. Our technique using ICG is briefly outlined as follows: we use an admixture of 10 mL of sterile water in a 25 mg bottled powder of ICG. Peritumoral injection of 0.5 mL of the ICG solution is performed via electromagnetic navigational bronchoscopy. The injection catheter is flushed with 0.5 mL of normal saline. The remaining 9.5 mL of ICG solution is stored and later given intravenously by the anesthesiologist after control and ligation of the segmental pulmonary artery(s) to help identify the intersegmental plane.
Conclusions
The take-home message from this well-written and well-performed study by the esteemed authors from Arizona and Florida is: robotic segmentectomy is safe, feasible and has now set a high bar for thoracic oncologic care. Other non-surgical therapies for NSCLC that are coming quickly and with great enthusiasm such as robotic bronchoscopic ablation therapy should be compared to minimally-invasive lung-sparing surgery [video-assisted thoracoscopic surgery (VATS) or robotic segmentectomy] rather than robotic, VATS or open lobectomy.
There are few if any 30- or 90-day mortalities with robotic segmentectomy. The operation can be safely done in under 2 h, most patients can go home within 23 h of surgery, the cancer-specific survival is high and the patient risk is very low. Perhaps most importantly—surgery has the advantage of providing a truly accurate stage of the patient. This allows those patients that may benefit from adjuvant therapy to be identified and treated more rapidly. Finally, and most importantly, surgical resection of the mass with lymphadenectomy provides the entire tumor—not just a random sample of it—to be analyzed for genetic and biological markers which are now a necessary part of our patients’ personalized cancer care.
Acknowledgments
None.
Footnote
Conflicts of Interest: Dr. Ferrari-Light has no disclosures or conflicts of interest to declare. Dr. Cerfolio discloses relationships with Bovie, Community Health Services, Covidien/Medtronic, C-SATS, Davol/Bard, Ethicon, Google/Verb, Intuitive Surgical, KCI/Acelity Company, Myriad Genetics, Pinnacle, ROLO-7 Consulting Firm and TEGO Corporation.
References
- Nguyen D, Gharagozloo F, Tempesta B, et al. Long-term results of robotic anatomical segmentectomy for early-stage non-small-cell lung cancer. Eur J Cardiothorac Surg 2019;55:427-33. [Crossref] [PubMed]
- Cerfolio RJ, Watson C, Minnich DJ, et al. One Hundred Planned Robotic Segmentectomies: Early Results, Technical Details, and Preferred Port Placement. Ann Thorac Surg 2016;101:1089-95; Discussion 1095-6. [Crossref] [PubMed]
- Geraci TC, Ferrari-Light D, Kent A, et al. Technique, Outcomes with Navigational Bronchoscopy Using Indocyanine Green for Robotic Segmentectomy. Ann Thorac Surg 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Cerfolio RJ, Ghanim AF, Dylewski M, et al. The long-term survival of robotic lobectomy for non-small cell lung cancer: A multi-institutional study. J Thorac Cardiovasc Surg 2018;155:778-86. [Crossref] [PubMed]
- Cerfolio RJ, Steenwyk BL, Watson C, et al. Decreasing the Preincision Time for Pulmonary Lobectomy: The Process of Lean and Value Stream Mapping. Ann Thorac Surg 2016;101:1110-5. [Crossref] [PubMed]
- Wei B, Cerfolio R. Technique of robotic segmentectomy. J Vis Surg 2017;3:140. [Crossref] [PubMed]
- Otsuka T, Nakamura Y, Harada A, et al. Extremely rare but potential complication of diffuse brain edema due to air embolism during lung segmentectomy with selected segmental inflation technique by syringe needle during video-assisted thoracoscopic surgery. J Thorac Cardiovasc Surg 2011;142:e151-2. [Crossref] [PubMed]
- Kiribayashi M, Nakasone M, Moriyama N, et al. Multiple cerebral infarction by air embolism associated with remarkable low BIS value during lung segmentectomy with video assisted thoracic surgery (VATS) technique: a case report. Masui 2010;59:480-3. [PubMed]


