
miR-19b-3p promotes human pancreatic cancer Capan-2 cells proliferation by targeting phosphatase and tension homolog
Introduction
Pancreatic carcinoma (PC) is a leading cause of cancer death worldwide, with the accelerating incidence and mortality in recent years. An estimated 37,685 new cases were diagnosed in United States annually (1). With the increasing complexity of the risk factors, the incidence of PC is rising. Its advanced stage at diagnosis and insensitivity to current therapies makes PC having the poorest prognosis of any major tumor types (2-4). Accordingly, it is imperative to explore new therapeutic targets for PC.
MicroRNAs (miRs) are short (∼21 nt in length) non-coding RNAs, which suppress target gene expression by base pairing with complementary nucleotides in the 3'untranslated region (3'UTR) to induce messenger RNA (mRNA) silence or translational inhibition (5). MiRs are involved in numerous pathological activities, including the development and progression of tumors (6). Some miRs are reported to participate in the genesis of pancreatic cancer. Downregulation of miR-192 could promote pancreatic cancer progression (7). miR-222, miR-182, and miR-454 have been discovered as potential regulatory factors for pancreatic cancer (8-11). Upregulation of miR-19b-3p has been reported in many types of cancers, including lung cancer (12), nasopharyngeal carcinoma (13), and breast cancer. However, the role of miR-19b-3p in pancreatic cancer is unknown. Phosphatase and tension homolog (PTEN) is a well-known target gene of miR-19b-3p involved in many tumorigenesis process (14), and the lipid phosphatase function of PTEN is critical for its anti-tumor activity.
Here we investigated the role of miR-19b-3p in controlling cell proliferation of human pancreatic cancer cell line Capan-2 cells. miR-19b-3p was found to be able to promote Capan-2 cells proliferation while miR-19b-3p inhibition decreased that. PTEN was identified as a target gene of miR-19b-3p, mediating the pro-proliferation effects of miR-19b-3p in Capan-2 cells.
Methods
Cell preparation
Human pancreatic cancer cells Capan-2 were acquired from the Shanghai Cell Bank of Chinese Academy of Sciences. RIPM1640 (Corning, USA) containing 10% fetal bovine serum (BI, USA) with penicillin (100 U/mL) and streptomycin (100 µg/mL) were used as a growth medium for Capan-2 cells culture. The cell cultures were maintained under a standard condition (37 °C, humidified atmosphere, 5% CO2).
miR and plasmid transfection
miR-19b-3p mimic and inhibitor were bought from RiboBio company (China). Two hundred thousands/mL cultured Capan-2 cells were plated into culture plates and allowed to attach overnight. After pretreated for 6 h with starvation culture medium (RIPM1640 without serum), transfection was conducted using Lipo2000 (Thermo, USA) complying with the manufacturer’s instructions, with miR-19b-3p mimic (50 nM), inhibitor (100 nM) or their negative controls for 48 h. PTEN overexpression plasmid was also transfected into Capan-2 cells with Lipo2000 (Thermo, USA).
5-ethynyl-2'-deoxyuridine (EdU) staining assay
Capan-2 cells were incubated with starvation medium containing 50 nM EdU (Life, China) for 2 h before the endpoint of the experiment. Capan-2 cells were fixed in 4% polyformaldehyde (PFA) at room temperature for 1 h. Then cells were incubated with 0.5% Triton X-100 for 15 min. Finally, cells were stained by Cell-Light™ EdU Cell Proliferation Detection Assay (Life, USA) according to the operating instruction. Numbers of EdU-positive and 4',6-diamidino-2-phenylindole (DAPI)-positive cells (counterstain) were counted under fluorescent microscope (Nikon, Germany). Ratio of proliferative cells were calculated by the ratio of EdU positive cells to DAPI positive cells.
Flow cytometry cell cycle analysis
At the end of transfection, cells were collected using 0.25% trypsin, fixed in 75% ethanol at −20 °C overnight. Propidium iodide (PI) staining (Sigma, USA) was performed to show cellular DNA content. Flow cytometry analysis was performed by using MoFlo XDP Cell Sorter (Beckman, USA). Proportion at different phases of cell cycle was analyzed using FlowJo7.6 software (Treestar Inc., USA).
Western blotting
RIPA Lysis Buffer (Thermo Fisher Scientific, USA) mixing with 1% phenylmethanesulfonyl fluoride (PMSF) was added to Capan-2 cells to extract total cell protein. Equal amounts of proteins were submitted to electrophorese on 8–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, transferred to nitrocellulose membranes and immunoblotted with the primary antibodies overnight. Polyclonal antibody GAPDH (Bioworld, USA; 1:5,000 dilution) and anti-PTEN (Abclonal, USA; 1:1,000 dilution) were used for immunoblotting. After immersed with the corresponding horseradish peroxidase (HRP)-coupled secondary antibodies for 3 h at room temperature, the membrane was washed thoroughly. Nitrocellulose membranes biding specific antibodies were visualized using enhanced chemiluminescence (ECL) system (Thermo Pierce, USA) with the Chemiluminescence imaging system (Bio-Rad, USA). The quantification of protein bands was performed using Image J. The expression level of target protein band was normalized by GAPDH protein band density.
Real-time fluorescence quantitative polymerase chain reaction (qRT-PCR)
RNA in cells was extracted by chloroform-isopropanol method and NanoDrop was used to determine the concentration of RNA. 400 ng RNA was converted into cDNA by iScripTM cDNA Synthesis Kit (Bio-Rad). The obtained cDNA was diluted 60 times and applied to quantitative PCR (qRT-PCR). Premix Ex TaqTM II (Perfect Real Time) in CFX384TM qRT-PCR Detection System (Bio-Rad) was used for detection. The temperature and time were pre-denaturing at 95 °C for 3 min, then 95 °C for 15 s, 60 °C for 30 s, 72 °C for 30 s. The cycle was repeated for 40 times. 18S RNA and 5S ribosomal RNA (5S rRNA) were applied as internal reference for mRNA and miR normalization, respectively. The primers used were derived from primerbank (https://pga.mgh.harvard.edu/primerbank/). PTEN, Froward: 5'-TGGATTCGACTTAGACTTGACCT-3', Reverse: 5'-GGTGGGTTATGGTCTTCAAAAGG-3'. 18S, Froward: 5'-TCAAGAACGAAAGTCGGAGG-3', Reverse: 5'-GGACATCTAAGGGCATCAC-3'Relative expression levels were determined using the 2‒ΔΔCt method.
Statistical analysis
Statistical analysis was carried out with SPSS statistics 20.0 (IBM, USA). The data were expressed as mean ± standard error of the mean (SEM). P values were calculated using tests for parametric, and an independent Student t test was used to compare two groups of experimental data, while the one-way ANOVA was used to analyze the results in multiple groups. If there were significant differences among three or more groups, Bonferroni’s test was performed to distinguish groups with significant differences. A significance value of P value <0.05 was set for all tests.
Results
miR-19b-3p promoted Capan-2 cells proliferation and cell cycle progression.
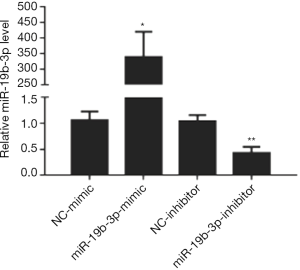
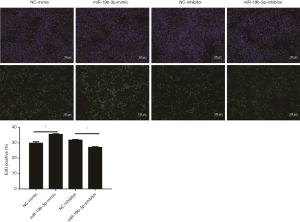
To detect the effects of miR-19b-3p in the proliferation of Capan-2 cells, miR-19b-3p mimic, inhibitor, and their negative controls were transfected. miR-19b-3p was found to be up-regulated after transfected with miR-19b-3p mimic, and down-regulated after transfected with miR-19b-3p inhibitor (Figure 1). After that, as determined by EdU incorporation assay, miR-19b-3p significantly raised the percentage of EdU-positive proliferating cells, while down-regulated miR-19b-3p decreased the percentage of EdU-positive proliferating cells (Figure 2). Consistently, flow cytometry showed that miR-19b-3p overexpression increased the percentage of cells in S phase while miR-19b-3p inhibitor decreased that (Figure 3). Collectively, our data confirmed that miR-19b-3p promoted Capan-2 cells proliferation.

PTEN was a target gene of miR-19b-3p in Capan-2 cells
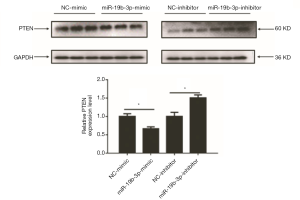
Whether PTEN is a target gene of miR-19b-3p in Capan-2 cells remains undetermined. By western blotting, it was found that miR-19b-3p mimic downregulated PTEN while miR-19b-3p inhibitor upregulated PTEN in Capan-2 cells. This data suggested that miR-19b-3p regulated PTEN at least at the protein level in Capan-2 cells (Figure 4).
PTEN mediated the effects of miR-19b-3p in regulating Capan-2 cells proliferation
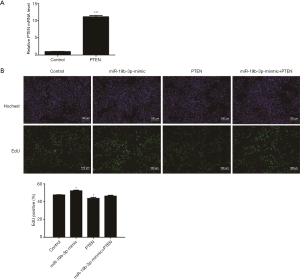
We further evaluated whether PTEN mediate the miR-19-3p-induced proliferation of Capan-2 cells. Firstly, we used qRT-PCR to confirm that PTEN overexpression plasmid successfully increased PTEN expression level at least at mRNA level (Figure 5A). Secondly, we found that PTEN overexpression eliminated the pro-proliferation effects of miR-19b-3p mimic in Capan-2 cells (Figure 5B). These data suggested that PTEN was a target gene of miR-19b-3p, mediating the effects of miR-19b-3p in regulating Capan-2 cells proliferation.
Discussion
Pancreatic cancer is one of the aggressive cancers featured by high disease morbidity and mortality (15). It is characteristic of recurrence and metastasis in early stage, and resistance to chemotherapeutics drug and radiotherapy, which make it one of the lethal diseases (16). Recently development of a variety of drugs including cell cycle inhibitors are under progression (17), however, the prognosis of pancreatic cancer patients has not been significantly improved. Therefore, further exploring the molecular regulatory mechanism in the occurrence of pancreatic cancer is of great significance for the treatment of pancreatic cancer.
Aberrantly expressed miRs contribute to many biological and pathological processes including cell growth, initiation and progression, apoptosis (18), which indicated the crucial role of miRs in tumor growth and development. miR-19b-3p can be generated by two kinds of stem loop. miR-19b-1 is leaguer of the miR-17-92 cluster, and miR-19b-2 belongs to miR-106-363 clusters. miR-19b-3p has been reported to control cell growth and survival (12,19). miR-19b-3p serves as an oncogene in multiple types cancers, including breast cancer, ovarian cancer, non-small cell lung cancer and colorectal cancer. The role of miR-19b-3p in tumor metastasis and angiogenesis, which is also crucial for tumor development, has also been reported (10,20). Especially, miR-19b-3p could regulate the differentiation and secretory function of β-cells (21), however the expression and contribution of miR-19b-3p in pancreatic cancer are still uncharted.
PTEN deleted on chromosome 10 has been characterized as a negative regulatory factor of the phosphoinositide 3-kinase (PI3K)-akt serine/threonine kinase 1 (AKT) signaling pathway. The most widely discussed anti-tumor activity of PTEN is its lipid phosphatase function (22-24). These could abnormally enhance the signaling, and thereby accelerate tumor cell growth, promote resistance to apoptosis and tumor progression. As a precancerous lesion of pancreatic ductal adenocarcinomas, intraductal papillary mucinous neoplasias (IPMNs) are developed in ductal cell-specific PTEN deficient mice, and the lesions have a more pronounced tendency to progress toward adenocarcinomas. These effects may or may not depend on the occurrence of KRAS proto-oncogene (K-RAS) activation. In IPMN patients, absence of PTEN in situ means poor prognosis and prone to malignant tumors. Besides, proliferation, invasion and mesenchymal-epithelial transition of human pancreatic cancer cells are inducted through PTEN/PI3K/AKT signaling (25,26). PTEN is a reported target gene of miR-19b-3p in other types of cells (27,28). In this study, we further confirmed the negative regulations of miR-19b-3p and PTEN in human pancreatic cancer cells. The functional rescue experiment showed that PTEN overexpression eliminated the pro-proliferation effects of miR-19b-3p mimic in Capan-2 cells. Interesting, some other target genes of miR-19b-3p have been reported, such as glycogen synthase kinase 3 beta (GSK3β), BRCA2 DNA repair associated (BRCA2), and their roles in miR-19b-3p-induced pancreatic cancer cells proliferation need further investigation (20). Nevertheless, this study demonstrates that PTEN is a target gene of miR-19b-3p in Capan-2 cells. Several limitations of the present study should be highlighted. Firstly, different types of pancreatic cancer cell line are required to strength the evidence for the effect of miR-19b-3p on pancreatic cancer cells. Secondly, the expression of miR-19b-3p and its target gene in human pancreatic cancer tissues remains unclear, which can provide more information for understanding the role of miR-19-3p in pancreatic cancer progression.
Conclusions
In conclusion, our study demonstrates that miR-19b-3p promotes Capan-2 cells proliferation by targeting PTEN.
Acknowledgments
Funding: This work was supported by the grants from National Natural Science Foundation of China (81820108006, 81670571 and 81370559 to C Yang), Joint Projects in Major Diseases funding from Shanghai Municipal Commission of Health and Family Planning (2014ZYJB0201 to C Yang), Joint Projects for Novel Frontier Technology in Shanghai Municipal Hospital from Shanghai Municipal Commission of Health and Family Planning (SHDC12014122 to C Yang), and funds from Shanghai Innovation Program (12431901002 to C Yang).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet 2016;388:73-85. [Crossref] [PubMed]
- E JY. Differential and Joint Effects of Metformin and Statins on Overall Survival of Elderly Patients with Pancreatic Adenocarcinoma: A Large Population-Based Study. Cancer Epidemiol Biomarkers Prev 2017;26:1225-32. [Crossref] [PubMed]
- Xu J, Gao C, Lee JKW, et al. PM2.5: A barrier to fitness and health promotion in China. J Sport Health Sci 2017;6:292-4. [Crossref] [PubMed]
- Wu S, Luo Y, Qiu X, et al. Building a healthy China by enhancing physical activity: Priorities, challenges, and strategies. J Sport Health Sci 2017;6:125-6. [Crossref] [PubMed]
- Liu Z, Wang J, Cheng H, et al. Cryo-EM Structure of Human Dicer and Its Complexes with a Pre-miR Substrate. Cell 2018;173:1191-203.e12. [Crossref] [PubMed]
- Profumo V, Forte B, Percio S, et al. LEADeR role of miR-205 host gene as long noncoding RNA in prostate basal cell differentiation. Nat Commun 2019;10:307. [Crossref] [PubMed]
- Botla SK, Savant S, Jandaghi P, et al. Early Epigenetic Downregulation of microRNA-192 Expression Promotes Pancreatic Cancer Progression. Cancer Res 2016;76:4149-59. [Crossref] [PubMed]
- Lee C, He H, Jiang Y, et al. Elevated expression of tumor miR-222 in pancreatic cancer is associated with Ki67 and poor prognosis. Med Oncol 2013;30:700. [Crossref] [PubMed]
- Zhao Y, Wang Y, Yang Y, et al. MicroRNA-222 Controls Human Pancreatic Cancer Cell Line Capan-2 Proliferation by P57 Targeting. J Cancer 2015;6:1230-5. [Crossref] [PubMed]
- Wang S, Ji J, Song J, et al. MicroRNA-182 promotes pancreatic cancer cell proliferation and migration by targeting beta-TrCP2. Acta Biochim Biophys Sin (Shanghai) 2016;48:1085-93. [Crossref] [PubMed]
- Fan Y, Shi C, Li T, et al. microRNA-454 shows anti-angiogenic and anti-metastatic activity in pancreatic ductal adenocarcinoma by targeting LRP6. Am J Cancer Res 2017;7:139-47. [PubMed]
- Li J, Yang S, Yan W, et al. MicroRNA-19 triggers epithelial-mesenchymal transition of lung cancer cells accompanied by growth inhibition. Lab Invest 2015;95:1056-70. [Crossref] [PubMed]
- Huang T, Yin L, Wu J, et al. MicroRNA-19b-3p regulates nasopharyngeal carcinoma radiosensitivity by targeting TNFAIP3/NF-kappaB axis. J Exp Clin Cancer Res 2016;35:188. [Crossref] [PubMed]
- Liu WG, Han LL, Xiang R. Protection of miR-19b in hypoxia/reoxygenation-induced injury by targeting PTEN. J Cell Physiol 2019. [Epub ahead of print]. [PubMed]
- Frič P, Škrha J, Šedo A, et al. Precursors of pancreatic cancer. Eur J Gastroenterol Hepatol 2017;29:e13-8. [Crossref] [PubMed]
- Mokdad AH, Dwyer-Lindgren L, Fitzmaurice C, et al. Trends and Patterns of Disparities in Cancer Mortality Among US Counties, 1980-2014. JAMA 2017;317:388-406. [Crossref] [PubMed]
- Franco J, Balaji U, Freinkman E, et al. Metabolic Reprogramming of Pancreatic Cancer Mediated by CDK4/6 Inhibition Elicits Unique Vulnerabilities. Cell Rep 2016;14:979-90. [Crossref] [PubMed]
- Wang L, Lv Y, Li G, et al. MicroRNAs in heart and circulation during physical exercise. J Sport Health Sci 2018;7:433-41. [Crossref] [PubMed]
- Jiang S, Li C, Olive V, et al. Molecular dissection of the miR-17-92 cluster's critical dual roles in promoting Th1 responses and preventing inducible Treg differentiation. Blood 2011;118:5487-97. [Crossref] [PubMed]
- Mogilyansky E, Clark P, Quann K, et al. Post-transcriptional Regulation of BRCA2 through Interactions with miR-19a and miR-19b. Front Genet 2016;7:143. [Crossref] [PubMed]
- Zhang ZW, Zhang LQ, Ding L, et al. MicroRNA-19b downregulates insulin 1 through targeting transcription factor NeuroD1. FEBS Lett 2011;585:2592-8. [Crossref] [PubMed]
- Malaney P, Uversky VN, Dave V. PTEN proteoforms in biology and disease. Cell Mol Life Sci 2017;74:2783-94. [Crossref] [PubMed]
- Benitez JA, Ma J, D'Antonio M, et al. PTEN regulates glioblastoma oncogenesis through chromatin-associated complexes of DAXX and histone H3.3. Nat Commun 2017;8:15223. [Crossref] [PubMed]
- Ying H, Elpek KG, Vinjamoori A, et al. PTEN is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-kappaB-cytokine network. Cancer Discov 2011;1:158-69. [Crossref] [PubMed]
- Ciuffreda L, Falcone I, Incani UC, et al. PTEN expression and function in adult cancer stem cells and prospects for therapeutic targeting. Adv Biol Regul 2014;56:66-80. [Crossref] [PubMed]
- Ma J, Sawai H, Ochi N, et al. PTEN regulates angiogenesis through PI3K/Akt/VEGF signaling pathway in human pancreatic cancer cells. Mol Cell Biochem 2009;331:161-71. [Crossref] [PubMed]
- Zhong C, Wang K, Liu Y, et al. miR-19b controls cardiac fibroblast proliferation and migration. J Cell Mol Med 2016;20:1191-7. [Crossref] [PubMed]
- Mavrakis KJ, Van Der Meulen J, Wolfe AL, et al. A cooperative microRNA-tumor suppressor gene network in acute T-cell lymphoblastic leukemia (T-ALL). Nat Genet 2011;43:673-8. [Crossref] [PubMed]


