Epidemiological, biological and clinical update on exercise-induced hemolysis
Introduction
Hemolysis is conventionally defined as a process of rupture and destruction of erythrocytes occurring either within (e.g., in vivo, also known as “intravascular hemolysis” or “hemolytic anemia”) or outside (i.e., in vitro, also known as “spurious hemolysis”) the circulation (1). Although the “normal” (i.e., physiological) concentration of free hemoglobin is typically comprised within 0.22–0.25 g/L in serum and between 0.10–0.13 g/L in plasma (2), the definition of hemolysis is usually reserved to conditions when serum or plasma samples contain a cell-free hemoglobin concentration >0.5 g/L and display a pink to slightly red color. When the concentration of cell-free hemoglobin exceeds 2–3 g/L, it is frequently referred to as “gross” or “frank” hemolysis, and is accompanied by red to brown color of serum or plasma, depending on final amount of protein (1).
The potential causes of intravascular hemolysis are many and multifaceted, and can be basically differentiated between congenital (e.g., hemoglobinopathies, disorders of erythrocyte membrane or metabolism) or acquired (e.g., immune-mediated, severe trauma or burns, hypersplenism, infections, mechanical intravascular injuries, incompatible blood transfusion or toxic) (1). Laboratory testing plays a substantial role, wherein intravascular hemolysis is conventionally diagnosed by some simple but straightforward findings, encompassing increased concentration of free hemoglobin in serum or plasma (as a direct index of erythrocyte destruction) (3), reduced values of haptoglobin (haptoglobin binds hemoglobin into the circulation to generate haptoglobin-hemoglobin complexes, which are then cleared by binding to specific receptors on macrophage surface) (4). Additional tests can be used for supporting the diagnosis of hemolytic anemia, such as the measurement of biomarkers of cytolysis (i.e., lactate dehydrogenase, aspartate aminotransferase, unconjugated bilirubin, potassium) or bone marrow stimulation (i.e., reticulocytes) (1), but they are plagued by an expectedly lower specificity.
According to the most recent statistics of Statista, one of the widest online statistics portals (5), running is the most popular and practiced sports worldwide, with as many as 60 million people engaged in some forms of jogging or running in the US (~18.4% of the entire resident population), and nearly half of these covering over 42 km each week. There is now incontrovertible evidence that a physically active lifestyle determines immense health benefits by lowering the risk of severe and frequently disabling pathologies such as cardiovascular disease, diabetes, osteoporosis, sarcopenia, frailty, and cancer (6,7). On the other hand, the regular practice of physical exercise may also have some unfavorable consequences such as sports injuries involving muscles, tendons and ligaments, accidental falls, bone fractures, cardiac arrhythmias, up to sudden death (8-10). Not only the musculoskeletal system may be injured by unaccustomed, incorrectly performed or extreme exercising, whereby several other organs and tissues may be affected, especially kidneys (11), liver (12) and bladder (13). Alongside the identification of possible unfavorable biological consequences, the main purposes of this article are: (I) establishing whether endurance exercise may be associated with intravascular hemolysis; (II) defining the possible determinants of exercise-induced hemolysis; and (III) reviewing the possible physiopathological mechanisms by which endurance exercise may promote, trigger or amplify intravascular hemolysis. In order to respond to these important questions, we have carried out a large free literature search for identifying all relevant studies that have previously addressed this problem and have summarized them in the following section of this narrative review.
Epidemiologic association between endurance exercise and intravascular hemolysis
The first case of probable exercise-induced hemolysis was described in 1884 by Kast (14). The German physician reported the case of a 19-year-old patient who developed gross hemoglobinuria after prolonged marches. Notably, he also observed that the lower were the periods of rest between consecutive marches, the shorter was the distance necessary to trigger hemoglobinuria. More than 30 years later, in 1916, the English Captain Macmanus described three cases of young men in early stage of military training who also developed gross hemoglobinuria after marching (15). The urine of the three young men appeared dark brown, with only few red blood cells (RBC). Spectroscopic analysis revealed that the color of the urine was attributable to large presence of methemoglobin. Notably, Macmanus attributed that unusual phenomenon to a combined effect of exercise and low temperature to which all the three cadets had been exposed. These two original reports then paved the way to the publication of a very large series of studies aimed at investigating the real burden of exercise-induced intravascular hemolysis.
The very first structured investigation on the relationship between exercise and hemolysis was published in 1943 by Gilligan and colleagues (16). The authors studied 11 male subjects who completed a cross-country run of 4.2–4.5 km, 11 male subjects who completed a cross-country run of 7.2–8.2 km and 22 male subjects who completed a long distance run of 42.2 km. An additional subject underwent a marathon, a 5-hour exercise on a cycle ergometer and a 421.6-km walk with a 24 kg pack on his back. Overall, 5/11 subjects (45.4%) displayed clearly reddish plasma with enhanced plasma hemoglobin values (i.e., >0.09 g/L) after both the 4.2–4.5 km (mean value, 0.08±0.03 g/L) and 7.2–8.2 km cross-country runs (mean value, 0.14±0.13 g/L), whilst this percentage increased to 81.8% (18/22) after the long-distance run (mean plasma hemoglobin value, 0.14±0.09 g/L). No correlation was found between plasma hemoglobin and age or training status. The concentration of hemoglobinuria, measured with benzidine reaction, was negative in all post-exercise urine samples after the 4.2–4.5 km cross-country run, was positive in 1 of the 8 subjects (12.5%) who provided urine after the 7.2–8.2 km cross-country run, and was positive in 4 of the 22 (18.2%) of those who completed the long-distance run.
In a subsequent investigation, Flatmark studied a 54-year-old man with previous history of exercise-induced hemoglobinuria (17). He was asked to exercise on a treadmill at 2 km/h for 180 min, at 4 km/h for 180 min or on a cycle ergometer for 120 min (17). Notably, plasma hemoglobin very modestly increased after exercising on treadmill at 2 km/h for 180 min or on the cycle ergometer, whilst it increased by over 6-fold after exercising on treadmill at 4 km/h.
An interesting study was published in 1964 by Davidson (18). Four subjects were asked to complete a 4.8 km run on two different grounds, i.e., grass or road. The concentration of plasma hemoglobin increased significantly after running on both types of grounds, but the relative increase was much higher after running on road (i.e., 6.0±4.8 folds) than on grass (i.e., 1.4±0.2 folds). Hemoglobinuria was absent in all four urine samples after grass running, whilst it was present in 1/4 (25%) urine samples after road running.
Some years afterwards, in 1979, Poortmans and Haralambie designed a comprehensive study based on assessment of a large number of biochemical parameters in 11 male athletes who were engaged in 100-km running (19). The values of serum hemoglobin consistently increased from 0.03±0.01 to 0.7±0.02 g/L at the end of the run. Serum haptoglobin values were instead found to be dramatically decreased by nearly 2.5-fold (i.e., from 1.19±0.65 to 0.47±0.45 g/L; P<0.001) immediately after the run, and remained significantly decreased compared to baseline (0.99±0.38 g/L; P<0.05) the day after the run.
Dufaux et al. carried out a cross-sectional study, including 62 male sedentary controls, 81 male professional rowers, 61 male middle- and long-distance runners, and 52 male professional cyclists (20). The concentration of serum haptoglobin was 1.0±0.3 g/L in the sedentary controls and was non-significantly different from that of the rowers (1.2±0.4 g/L) or professional cyclists (1.1±0.2 g/L), whilst that of the runners was significantly lower compared with the other groups (0.7±0.4 g/L; P<0.01).
Hunding and coworkers measured plasma hemoglobin and haptoglobin in 3 trained distance runners who performed a 25-km run (21). Plasma hemoglobin increased in all three subjects from 0.03±0.03 to 0.43±0.13 g/L (i.e., 12-fold increase), whilst plasma haptoglobin remained almost unchanged. All three subjects had plasma hemoglobin values over the upper reference limit (URL) (>0.2 g/L) at the end of the run.
Falsetti et al. carried out another interesting study, including 23 male marathon runners, who were randomly divided in two groups according to the type of shoes worn for running (i.e., 11 subjects wearing firm-sole and 12 subjects wearing soft air-sole shoes) (22). Plasma hemoglobin and haptoglobin concentration measured before and after a 24-km run were 0.05±0.04 vs. 0.08±0.08 g/L and 0.45±0.47 vs. 0.34±0.46 g/L in the firm-sole shoe group, thus exhibiting a 1.37-fold increase and a 1.34-fold decrease, respectively. Conversely, plasma hemoglobin and haptoglobin concentration measured before and after a 24-km run were 0.03±0.01 vs. 0.04±0.01 g/L and 0.50±0.24 vs. 0.47±0.28 g/L in the firm-sole shoe group, thus exhibiting a more limited 1.15-fold increase and 1.06-fold decrease, respectively.
Eichner performed an interesting experiment, in which a middle-aged man was regularly monitored after his training periods by means of plasma haptoglobin measurements (23). Notably, a strong inverse correlation was found between running distance and variation of this biomarker (r=−0.91).
Nyman studied 19 runners (16 men and 3 women) who ran on average 16.1 km/day each for covering a total distance of 1,207 km (24). Before the start of the trial, plasma haptoglobin levels were lower than the URL in 5/19 athletes (26.3%), but in 15 of them (78.9%) the values progressively decreased during the run.
Davidson et al. measured plasma haptoglobin in 115 runners (90 men and 25 women) before and immediately after the end of a competitive marathon (25). In both male (0.80±0.65 vs. 1.15±0.66 g/L; P<0.001) and female (0.62±0.51vs 1.14±0.56 g/L; P<0.001) athletes, plasma haptoglobin values significantly declined at the end of the run, by 1.4- and 1.8-fold, respectively.
A similar study was carried out by Egan et al., who measured serum haptoglobin values in 8 male athletes before and after a marathon race (26). As predictable, the mean serum haptoglobin values substantially decreased by approximately 1.3-fold immediately after the run (0.97±0.48 vs. 1.29±0.18 g/L; P<0.05), and were found to be further reduced 6 hours afterwards (0.86±0.35 gL; P<0.05).
Wolf and colleagues studied 11 male prominent middle-distance runners who completed a 5-km run at their maximal effort (27). The serum haptoglobin value was found to be reduced by approximately 4.7-fold at the end of the trial (0.20±0.02 vs. 0.95±0.08 g/L; P<0.03).
Lijnen et al. investigated the variation of cell breakdown biomarkers in the plasma of 23 male runners who were engaged in a marathon race (28). Plasma haptoglobin values decreased from 1.20±0.15 g/L before the run to approximately 0.75±0.15 g/L immediately after, remained decreased during 12 hours (0.85±0.15 g/L), and only returned to the baseline pre-run values 72 hours after the end of the marathon.
Miller et al. planned an interesting experiment for exploring the potential association between foot impact force and changes of hemolysis biomarkers in 14 male distance runners (29). The athletes completed two treadmill run at 12.9 km/h for a total number of 10,000 foot-strikes at two different elevations (i.e., −6% or +6%; force +11% higher running downhill vs. uphill). Plasma hemoglobin increased from 0.023±0.010 to 0.051±0.023 g/L (i.e., 2.2-fold increase) and from 0.037±0.023 to 0.086±0.051 g/L (i.e., 2.3-fold increase) after running uphill and downhill, respectively. Likewise, plasma haptoglobin decreased from 0.42±0.28 to 0.37±0.27 g/L (1.1-fold decrease) and from 0.42±0.30 to 0.33±0.29 g/L (i.e., 1.3-fold decrease), respectively.
O’Toole and colleagues carried out a large study based on 95 athletes who participated in two triathlon races of different distances (30). Thirty athletes (11 men and 19 women) first completed a 1.5-km swimming, 40-km cycling and 10-km running distance, whilst 65 other athletes (46 men and 19 women) completed a 3.9-km swimming, 180-km cycling and 42.2-km running distance. Overall, 95% of all runners displayed a decrease of serum haptoglobin immediately after the races, with a mean 1.28-fold decrease. Although the percentage of athletes with decreased haptoglobin values was nearly similar between the two races (i.e., 95% vs. 93%), the relative haptoglobin decrease was higher in athletes who completed the longer race (i.e., 32% vs. 20%). The percentage of athletes with occult blood in urine was also higher after the longer race (31% vs. 23%).
Seiler et al. studied 110 well-trained athletes (91 men and 19 women) who enrolled in a 1000-km running competition, lasting for 20 days (average running per day, 50 km) (31). The authors measured serum haptoglobin in 51 of these subjects at different time points and found that mean serum haptoglobin value was systematically lower than the baseline until the 11th running day (the lowest values were found after the first day with a nearly 1.5-fold decrease), whilst the concentration returned to pre-run values at the end of the trial. Even more importantly, although only 5% of urine samples were positive for blood before the start of the run, the percentage of positive samples for blood was always >25% throughout the study period (the peak was 34.5%, as recorded at the end of the competition).
Deitrick carried out a study including 15 male recreational runners in whom circulating haptoglobin and urobilinogen levels were measured before, immediately after, 1 day, 4 days, and 10 days after a 13-km run (32). Immediately after the run serum haptoglobin non-significantly decreased from 0.92±0.69 to 0.80±0.75 g/L, whilst a statistically significant reduction was then noted 24 hours after the end of the run (0.66±0.66 g/L; P<0.05), with values remaining significantly decreased up to 4 days after the run (0.59±0.53 g/L; P<0.05). The values of urobilinogen were also significantly increased at 1 and 24 hours after the run.
Dressendorfer et al. investigated the impact of 7 consecutive days of prolonged jogging (2 h per day at ~80% of maximal heart rates) in 10 moderately fit men, who finally covered a total distance of 129 km (33). The mean haptoglobin level decreased from 0.86 to 0.67 g/L on day 5 (P<0.05), and further decreased to 0.61 g/L (P<0.05) at day 8.
Weight et al. studied 20 male runners who completed a 42-km marathon (34). The plasma hemoglobin value was found to be substantially increased immediately after the run (0.113±0.074 vs. 0.077±0.050 g/L), whilst the serum concentration of haptoglobin significantly decreased (0.69±0.40 vs. 0.89±0.40 g/L). The values of both plasma hemoglobin and haptoglobin returned to the baseline after 24 hours. Interestingly, the authors also showed that the mean RBC lifespan in distance runners (i.e., 67 and 72 days in men and women, respectively) was considerably shorter than in sedentary controls (i.e., 113 and 114 days in men and women, respectively).
In another interesting investigation, Dressendorfer and colleagues (35) randomly divided a group of 14 male runners into those wearing firm-sole or soft-sole shoes. All athletes were then asked to undergo increased distance training, completing 430 km in 17 days. Plasma haptoglobin decreased more in the soft-sole (from 0.55±0.09 to 0.43±0.07 g/L; −27%) than in the firm-sole (from 0.59±0.11 to 0.50±0.10 g/L; −15%) shoes group, whilst plasma hemoglobin also increased more in the soft-sole (from 0.08±0.09 to 0.14±0.03 g/L; 75%) than in the firm-sole (from 0.12±0.07 to 0.19±0.08 g/L; 58%) shoes group.
De Paz et al. studied 13 male runners who participated to a 100-km run (36). Serum haptoglobin considerably decreased from 0.66±0.18 g/L at baseline to 0.22±0.05 g/L immediately after the run.
Jordan et al. studied 13 male runners who engaged in a marathon race (37). Plasma haptoglobin decreased from approximately 1.2±0.8 g/L before the start of the marathon to 0.7±0.8 g/L immediately after. Another important aspect emerged from this study was that long-distance running induced significant RBC membrane skeletons modifications, probably due to a process of in vivo proteolysis.
An extreme exercise study was planned by Fallon et al. (38), who recruited 9 athletes (7 men and 2 women) participating in a 1,600-km ultramarathon. At variance with all the other studies, the authors found a substantial increase of serum haptoglobin from baseline (1.8±0.7 g/L) throughout (3.4±0.9 and 3.9±0.6 g/L) and after the run (4.1±1.2 g/L).
Schumacher et al. carried out another interesting study aimed at investigating several hematologic biomarkers in athletes of different sport disciplines (39). Interestingly, although no significant differences were observed in the concentration of serum haptoglobin between the whole cohorts of athletes (n=747; 0.67±0.37 g/L) and sedentary people (n=104; 0.67±0.40 g/L), runners (n=144; 0.50±0.35 g/L) displayed a substantially lower value than cyclists (n=272; 0.80±0.34).
Telford and coauthors published an interesting experiment, in which 10 male triathletes completed two different 1-hour sessions of cycling and running at 75% maximal oxygen consumption (40). Plasma hemoglobin values were found to be significantly increased after both the cycling (0.049±0.01 vs. 0.030±0.01) and running (0.120±0.02 vs. 0.037±0.01 g/L) sessions, but the relative increase was nearly double after running (3.2- vs. 1.6-fold). Haptoglobin values followed an inverse trend, with a much higher decrease after running than after cycling (the mean changes were 0.016 and 0.085 g/L after cycling and running, respectively).
Yusof et al. studied 6 male runners who engaged in a 216-km ultra-endurance race (41). Serum haptoglobin, which was measured at baseline (0.87±0.24 g/L) and at different time points throughout the run, showed a sharp decrease after 21 km (0.71±0.19 g/L) and 42 km (0.59±0.19 g/L), reaching its lowest value after 84 km (0.43±0.18 g/L). An opposite trend was then noted afterwards, with values slightly increasing after 126 km (0.46±0.27) and more markedly growing at the end of the run (0.64±0.34), thought remaining lower than at baseline. Interestingly, the authors also found that osmotic fragility was dramatically increased throughout the running distance.
Peeling et al. investigated the effect of two different training surfaces (i.e., grass or bitumen road) in 10 trained male runners, who performed two 10-km runs and ten 1-km interval running sessions (42). Interestingly, serum hemoglobin increased from 0.041±0.003 to 0.049±0.003 g/L after grass running (i.e., 19% increase) and from 0.032±0.003 to 0.044±0.002 g/L after road running (i.e., 40% increase), respectively. Plasma haptoglobin decreased from 0.58±0.12 to 0.51±0.11 g/L after grass running (i.e., 14% decrease) and from 0.66±0.13 to 0.58±0.12 g/L after road running (i.e., 14% decrease), respectively. The same group of authors also highlighted in another publication that the hemolysis degree was substantially similar after the 10-km run and the ten 1-km interval running sessions (both displaying a 37% increase of serum hemoglobin) (43).
Interesting findings have also been published by Sim et al., who measured serum hemoglobin and haptoglobin in 11 male athletes undergoing two 90-min running session at 75% of peak oxygen uptake, consuming either a 6% carbohydrate solution or placebo (44). The values of serum hemoglobin were found to be significantly increased decreased after both sessions, whilst those of haptoglobin were significantly decreased after both sessions. Notably, carbohydrate intake was effective to reduce the hemoglobin increase (1.60-fold vs. 1.86-fold for placebo) but not the haptoglobin decrease (1.27-fold versus 1.20-fold for placebo).
Gough et al. studied 18 male athletes who participated in an endurance triathlon race (3.8-km swimming, 180-km cycling and 42.2-km running distance) (45). Serum haptoglobin levels immediately after the race were substantially decreased compared to baseline (0.16 vs. 0.48 g/L).
Lippi and colleagues measured serum haptoglobin in 18 male runners who completed a 60-km ultramarathon (46), and found a substantial decrease of its values immediately after the end of the run (0.36 vs. 0.68 g/L).
Binnie et al. performed two separate investigations to assess the effect of different training surfaces on several biochemical parameters in 10 male athletes. In the first experiment the athletes completed two interval training sessions, one on soft dry beach sand and the other on well-maintained sporting grass ground (47). Each session consisted of three interval sets separated by 5 min of rest of dynamic running (2×45:90 s, 3×20:60 s and 2×15:45 s). After both sessions, the concentration of serum haptoglobin was found to be significantly decreased, though the absorptive qualities of sand were effective to slightly attenuate haptoglobin reduction compared to the grass (1.08-fold decrease in sand vs. 1.12-fold decrease in grass, respectively). The design of the second part of the experiment was more or less similar to the former, with two repeated sessions of sprint bouts, agility and power drills involving rapid changes of direction and speed (48). As in the former experiment, one session was performed on soft dry beach sand and the other on well-maintained sporting grass ground. Unlike previous findings, and although the concentration of serum haptoglobin was found to be significantly decreased after both sessions, such decrease was also partially attenuated by the absorptive qualities of sand (1.14-fold decrease in sand vs. 1.20-fold decrease in grass, respectively).
In a subsequent investigation Christensen et al. studied 10 male runners who took part in a 78-km race developing at 2,400 m above the sea level (49). Plasma haptoglobin values dramatically decrease from 1.48±0.30 g/L pre-run, to 0.74±0.31 g/L immediately after the run, and remained so up to 6 hours afterwards. Haptoglobin concentration returned to pre-run values after 24 hours.
Another interesting study was published by Robach et al. (50), who examined biochemical changes in 22 male runners after a 166-km ultra-marathon with 9,500 m of altitude gain/loss. Plasma hemoglobin increased from 0.12±0.57 g/L before the run to 0.14±0.85 immediately after, whilst serum haptoglobin concentration was found to be significantly decreased immediately after the end of the run (0.74±033 vs. 0.87±0.34 g/L). Notably, the values of both plasma hemoglobin and serum haptoglobin normalized the following day after the run. Interestingly, the total plasma hemoglobin content of blood was also found to be increased by nearly 1.4-fold at the end of the run (570±363 vs. 395±187 mg).
Chiu et al. studied 25 male athletes who completed a 100-km ultramarathon (51). Plasma hemoglobin was found to be slightly increased after the end of the run (from 0.3±0.2 to 0.4±0.5 g/L), whilst serum haptoglobin was considerably decreased (from 0.64±0.31 to 0.30±0.30). Both values normalized 24 hours after the end of the run.
Caulfield et al. studied 19 male runners who engaged in 8×3 min motorized treadmill exercises at 90% of maximal oxygen consumption (52). A significant reduction of serum haptoglobin was noted at the end of the trial, which was overall comparable among fore-foot (n=10; −9.3%) and rear-foot (n=9; −10.6%; P=0.902) athletes.
Finally, Liu et al. studied 19 male athletes who participated in a 24-hour ultramarathon (median distance covered, 154 km) (53). Unlike the vast majority of other studies, plasma hemoglobin concentration was found to be modestly decreased rather than increased at the end of the run (i.e., 0.2 vs. 0.3 g/L; P<0.001), a finding which was actually at odds with the remarkable decrease of serum haptoglobin contextually observed in the same cohort of athletes (0.19 vs. 0.77 g/L; P<0.001).
Possible causes of exercise-induce hemolysis
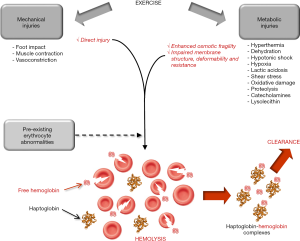
Once clearly established that exercise-induce hemolysis is a frequent phenomenon in athletes engaged in endurance exercise (especially runners), it seems reasonable to classify the potential (and often coexisting) causes into two leading categories, thus encompassing mechanical and metabolic factors (Figure 1). Indeed, a certain degree of direct erythrocyte injury cannot be denied as logic consequence of repeated foot impacts (especially heel strikes) on the ground. This is clearly deducible from studies showing that running on soft surfaces (i.e., on sand or grass) (18,42,47,48) or wearing cushioned shoe (i.e., soft air-sole shoes) (22) is sometimes effective to reduce but not completely neutralize exercise-induced hemolysis, as well as by other investigations demonstrating that the degree of hemolysis was dependent on intensity (29) or overall number (long distance or accelerated stepping cadence) (16,23,30) of ground impacts. On the other hand, reliable evidence attests that additional mechanisms other than foot strike may be involved in exercise-induced hemolysis. For example, Beneke et al. studied 10 male triathletes who engaged in two sessions of 35-min low- and high-intensity cycling, which does not involve any possible mechanical RBC trauma at foot strike (54). Interestingly, although low intensity cycling triggered a modest increase of plasma haptoglobin (from 1.30 to 1.49 g/L), high intensity cycling elicited a modest but significant reduction of this biomarker (from 1.10 to 1.01 g/L), confirming that even high intensity exercise per se is capable to trigger a certain degree of hemolysis independently from injuries caused by foot hitting the ground. Similar evidence has been published in other nontraumatic sport disciplines such as endurance swimming (55). The repeated contractile activity of muscles during exercise is then another mechanism which may cause erythrocyte mechanical injury, whereby RBC compression during powerful and repeated muscle contractions (especially within the capillary network) may finally results in their rupture. Sustained vasoconstriction of internal organs (especially kidneys), a physiological process aimed at deviating large part of blood flow into the exercising muscles, is another frequent phenomenon in endurance running (21,56), which may contribute to generate RBC compression and injury in smaller arteries.
Beside direct mechanical injuries, a kaleidoscope of other metabolic causes and mechanisms occurring while exercising may promote enhanced erythrocyte fragility and/or impaired membrane structure, deformability or resistance (especially for older RBC), finally contributing to promote or increase the chance of intravascular destruction (Figure 1). Enhanced catecholamines values, as commonplace in sports, interplay with specific receptors at RBC surface, cause impaired membrane deformability and ultimately promote higher vulnerability to hemolysis (57). A parallel mechanism has been described by Yamada et al., who showed that exercise adaptation induces a significant change of lipid profile, accompanied by an increase of lysolecithin and a reduction of free cholesterol in the erythrocyte membrane, two converging phenomenon which contribute to enhance osmotic fragility (58). The hypothesis that RBC membrane may be altered during exercise has been confirmed by Beneke et al. (54), who identified abnormalities in alpha- and beta-spectrin comparable to those found in congenital erythrocyte disorders (e.g., spherocytosis). This hypothesis has been further confirmed by others (41). Hyperthermia, which is commonplace in athletes engaged in strenuous and/or prolonged exercise, significantly enhance erythrocyte fragility and thereby their propensity to rupture (59). Acidosis is another important mechanism which may at least partially explain the association between longer distance and higher propensity to develop hemolysis. An increased production of lactic acid is frequent in endurance athletes, and the osmotic fragility of erythrocytes has been recently shown to increase in parallel with blood lactate concentration (60). Hypoxia, hypotonic shock and shear stress are additional conditions frequently developing in exercising muscles, and recent evidence has been provided that they may altogether contribute to strongly enhance ATP efflux from RBC, thus ultimately escalating their susceptibility to intravascular hemolysis (61). Reliable evidence has also been provided that exercise-induced dehydration may contribute to increase erythrocyte fragility in vivo, as demonstrated by Platt et al. (62). Smith et al. also showed that strenuous running is associated with a remarkable reduction of erythrocyte antioxidant capacity, making these cells much more vulnerable to lysis (63).
Although no systematic evidence is available in the scientific literature, it seems reasonable to hypothesize that patients bearing some underlying erythrocyte disorders causing increased osmotic fragility may be more vulnerable to developing exercise-induced hemolysis (Figure 1). Some paradigmatic cases have been described, such as those of patients with hereditary spherocytosis (64), sickle cell disease (65) or glucose-6-phosphate dehydrogenase (G6PD) deficiency (66), among others. Beside subjects carrying inherited or acquired erythrocyte disorders, which would increase per se the baseline risk of intravascular hemolysis, there are other conditions associated with enhanced erythrocyte fragility in vivo and may contribute to magnify the likelihood of exercise-induced hemolysis. These may actually include hyponatremia (67), diabetes (68) and chronic liver disease (69).
Conclusions
According to different pathogenetic mechanisms, the classical definition of foot-strike hemolysis seems now inappropriate for identifying all cases of intravascular hemolysis occurring while exercising. This is due to the fact that several studies showed that attenuating RBC injuries derived from ground contact is not completely effective for preventing intravascular hemolysis. Therefore, we suggest that foot-strike (or contact) hemolysis shall now be considered only a part of the more thoughtful and appropriate concept of exercise-induced hemolysis.
The literature data reviewed in this narrative review attests that a significant degree of exercise-induced hemolysis is commonplace after short-, medium-, long- and ultra-long distance running, as reflected by the significant decrease of serum or plasma haptoglobin combined with the significant increase of plasma hemoglobin concentration or overall blood content (Table 1). This paraphysiological intravascular hemolysis is typically mild (the average variations of hemolysis biomarkers are usually comprised between 1.2- and 1.8-fold), almost self-limiting (i.e., completely resolving within 24–48 hours), with relative extent depending on athlete population, analytical technique used for detecting intravascular hemolysis as well as on number, frequency and intensity of ground contacts, but not on running technique (i.e., fore-foot or rear-foot) (Table 1). Additional lines of evidence support the notion that both osmotic fragility and membrane structure of erythrocytes are considerably modified during endurance exercise (41), and this fact goes hand in hand with findings that erythrocyte lifespan in runners is approximately 40% shorter than in sedentary controls (e.g., 70 vs. 114 days) (34). Direct mechanical injury caused by forceful ground contacts, repeated muscle contractile activity or vasoconstriction in internal organs are three potential sources of exercise-induced hemolysis, whilst preexisting erythrocyte disorders and metabolic abnormalities developed while exercising (e.g., hyperthermia, dehydration, hypotonic shock, hypoxia, lactic acidosis, shear stress, oxidative damage, proteolysis, increased concentration of catecholamines and lysolecithin) may actively contribute to trigger, accelerate or amplify this phenomenon (Figure 1).
Full table
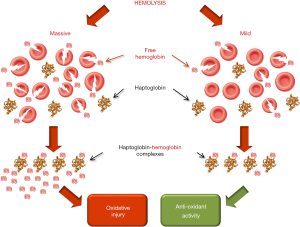
Regarding the possible clinical significance of mild and transitory intravascular hemolysis in athletes, an interesting study has revealed that cell-free hemoglobin levels are inversely correlated with mortality and hospital admissions for heart failure (i.e., each 0.1 g/L increase in plasma hemoglobin reduces the risk of mortality and heart failure-related hospital admissions of 40% and 21%, respectively) (71). Unlike severe hemolytic diseases, where massive release of cell-free hemoglobin overwhelms the potency of the homeostatic clearance systems and directly triggers a severe oxidative injury (72), the much lower concentration of cell-free hemoglobin generated from exercise-induced hemolysis is completely cleared by scavenger plasma proteins, especially haptoglobin. Within these complexes, the hemoglobin-related pseudoperoxidase activity is then transformed from detrimental into protective, ultimately contributing to safeguard cell integrity and offering protection to exercise-intensified oxidative stress (Figure 2) (73). This mechanism may hence be seen as a potentially beneficial pathway, which may ultimately contribute to amplify the many benefits of regular physical exercise in reducing the risk of morbidity, frailty and mortality (74-76).
Acknowledgments
F Sanchis-Gomar is supported by a postdoctoral contract granted by “Subprograma Atracció de Talent-Contractes Postdoctorals de la Universitat de València”.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lippi G, Plebani M, Di Somma S, et al. Hemolyzed specimens: a major challenge for emergency departments and clinical laboratories. Crit Rev Clin Lab Sci 2011;48:143-53. [Crossref] [PubMed]
- Lippi G, Giavarina D, Gelati M, et al. Reference range of hemolysis index in serum and lithium-heparin plasma measured with two analytical platforms in a population of unselected outpatients. Clin Chim Acta 2014;429:143-6. [Crossref] [PubMed]
- Lippi G, Favaloro EJ, Franchini M. Haemolysis index for the screening of intravascular haemolysis: a novel diagnostic opportunity? Blood Transfus 2018;16:433-7. [PubMed]
- Van Vlierberghe H, Langlois M, Delanghe J. Haptoglobin polymorphisms and iron homeostasis in health and in disease. Clin Chim Acta 2004;345:35-42. [Crossref] [PubMed]
- Statista. Running & Jogging - Statistics & Facts. Available online: https://www.statista.com/topics/1743/running-and-jogging/. Accessed May 2, 2019.
- Lippi G, Schena F, Guidi GC. Health benefits of physical activity. CMAJ 2006;175:776. [Crossref] [PubMed]
- Lippi G, Schena F. Run for Science (R4S): the history of a successful project of precision and laboratory medicine in sport and exercise. J Lab Precis Med 2017;2:11. [Crossref]
- Lippi G, Schena F, Ceriotti F. Diagnostic biomarkers of muscle injury and exertional rhabdomyolysis. Clin Chem Lab Med 2018;57:175-82. [Crossref] [PubMed]
- Perez-Quilis C, Lippi G, Cervellin G, et al. Exercising recommendations for paroxysmal AF in young and middle-aged athletes (PAFIYAMA) syndrome. Ann Transl Med 2017;5:24. [Crossref] [PubMed]
- Lippi G, Favaloro EJ, Sanchis-Gomar F. Sudden Cardiac and Noncardiac Death in Sports: Epidemiology, Causes, Pathogenesis, and Prevention. Semin Thromb Hemost 2018;44:780-6. [Crossref] [PubMed]
- Hodgson LE, Walter E, Venn RM, et al. Acute kidney injury associated with endurance events-is it a cause for concern? A systematic review. BMJ Open Sport Exerc Med 2017;3:e000093. [Crossref] [PubMed]
- Lippi G, Schena F, Montagnana M, et al. Significant variation of traditional markers of liver injury after a half-marathon run. Eur J Intern Med 2011;22:e36-8. [Crossref] [PubMed]
- Blacklock NJ. Bladder trauma in the long-distance runner: "10,000 metres haematuria Br J Urol 1977;49:129-32. [Crossref] [PubMed]
- Kast A. Ueber paroxysmale Hämoglobinurie durch Gehen. Dtsch med Wochenschr 1884;10:840-2. [Crossref]
- Macmanus D. Three Cases of Paroxysmal Haemoglobinuria in Soldiers. Br Med J 1916;1:552. [Crossref] [PubMed]
- Gilligan DR, Altschule MD, Katersky EM. Physiological Intravascular Hemolysis of Exercise. Hemoglobinemia and Hemoglobinuria Following Cross-Country Runs. J Clin Invest 1943;22:859-69. [Crossref] [PubMed]
- Flatmark T. Studies on the hemolytic mechanism in March hemoglobinuria. Acta Med Scand 1963;173:307-13. [Crossref] [PubMed]
- Davidson RJ. Exertional Haemoglobinuria: A Report on Three Cases with Studies on the Haemolytic Mechanism. J Clin Pathol 1964;17:536-40. [Crossref] [PubMed]
- Poortmans JR, Haralambie G. Biochemical changes in a 100 km run: proteins in serum and urine. Eur J Appl Physiol Occup Physiol 1979;40:245-54. [Crossref] [PubMed]
- Dufaux B, Hoederath A, Streitberger I, et al. Serum ferritin, transferrin, haptoglobin, and iron in middle- and long-distance runners, elite rowers, and professional racing cyclists. Int J Sports Med 1981;2:43-6. [Crossref] [PubMed]
- Hunding A, Jordal R, Paulev PE. Runner's anemia and iron deficiency. Acta Med Scand 1981;209:315-8. [Crossref] [PubMed]
- Falsetti HL, Burke ER, Feld RD, et al. Hematological Variations After Endurance Running With Hard-and Soft-Soled Running Shoes. Phys Sportsmed 1983;11:118-27. [Crossref] [PubMed]
- Eichner ER. Runner's macrocytosis: a clue to footstrike hemolysis. Runner's anemia as a benefit versus runner's hemolysis as a detriment. Am J Med 1985;78:321-5. [Crossref] [PubMed]
- Nyman CR. Haematological and biochemical observations during a 750 mile relay. Br J Sports Med 1985;19:156-7. [Crossref] [PubMed]
- Davidson RJ, Robertson JD, Galea G, et al. Hematological changes associated with marathon running. Int J Sports Med 1987;8:19-25. [Crossref] [PubMed]
- Egan LM, Watts PB, Silta BC. Changes in serum haptoglobin as an acute response to a marathon road race. J Sports Sci 1987;5:55-60. [Crossref] [PubMed]
- Wolf PL, Lott JA, Nitti GJ, et al. Changes in serum enzymes, lactate, and haptoglobin following acute physical stress in international-class athletes. Clin Biochem 1987;20:73-7. [Crossref] [PubMed]
- Lijnen P, Hespel P, Fagard R, et al. Indicators of cell breakdown in plasma of men during and after a marathon race. Int J Sports Med 1988;9:108-13. [Crossref] [PubMed]
- Miller BJ, Pate RR, Burgess W. Foot impact force and intravascular hemolysis during distance running. Int J Sports Med 1988;9:56-60. [Crossref] [PubMed]
- O'Toole ML, Hiller WD, Roalstad MS, et al. Hemolysis during triathlon races: its relation to race distance. Med Sci Sports Exerc 1988;20:272-5. [Crossref] [PubMed]
- Seiler D, Nagel D, Franz H, et al. Effects of long-distance running on iron metabolism and hematological parameters. Int J Sports Med 1989;10:357-62. [Crossref] [PubMed]
- Deitrick RW. Intravascular haemolysis in the recreational runner. Br J Sports Med 1991;25:183-7. [Crossref] [PubMed]
- Dressendorfer RH, Wade CE, Claybaugh J, et al. Effects of 7 successive days of unaccustomed prolonged exercise on aerobic performance and tissue damage in fitness joggers. Int J Sports Med 1991;12:55-61. [Crossref] [PubMed]
- Weight LM, Byrne MJ, Jacobs P. Haemolytic effects of exercise. Clin Sci (Lond) 1991;81:147-52. [Crossref] [PubMed]
- Dressendorfer RH, Wade CE, Frederick EC. Effect of shoe cushioning on the development of reticulocytosis in distance runners. Am J Sports Med 1992;20:212-6. [Crossref] [PubMed]
- De Paz JA, Villa JG, Lopez P, et al. Effects of long-distance running on serum bilirubin. Med Sci Sports Exerc 1995;27:1590-4. [Crossref] [PubMed]
- Jordan J, Kiernan W, Merker HJ, et al. Red cell membrane skeletal changes in marathon runners. Int J Sports Med 1998;19:16-9. [Crossref] [PubMed]
- Fallon KE, Sivyer G, Sivyer K, et al. Changes in haematological parameters and iron metabolism associated with a 1600 kilometre ultramarathon. Br J Sports Med 1999;33:27-31; discussion 32. [Crossref] [PubMed]
- Schumacher YO, Schmid A, Grathwohl D, et al. Hematological indices and iron status in athletes of various sports and performances. Med Sci Sports Exerc 2002;34:869-75. [Crossref] [PubMed]
- Telford RD, Sly GJ, Hahn AG, et al. Footstrike is the major cause of hemolysis during running. J Appl Physiol 2003;94:38-42. [Crossref] [PubMed]
- Yusof A, Leithauser RM, Roth HJ, et al. Exercise-induced hemolysis is caused by protein modification and most evident during the early phase of an ultraendurance race. J Appl Physiol (1985) 2007;102:582-6. [Crossref] [PubMed]
- Peeling P, Dawson B, Goodman C, et al. Training surface and intensity: inflammation, hemolysis, and hepcidin expression. Med Sci Sports Exerc 2009;41:1138-45. [Crossref] [PubMed]
- Peeling P, Dawson B, Goodman C, et al. Cumulative effects of consecutive running sessions on hemolysis, inflammation and hepcidin activity. Eur J Appl Physiol 2009;106:51-9. [Crossref] [PubMed]
- Sim M, Dawson B, Landers G, et al. The effects of carbohydrate ingestion during endurance running on post-exercise inflammation and hepcidin levels. Eur J Appl Physiol 2012;112:1889-98. [Crossref] [PubMed]
- Gough CE, Eastwood A, Saunders PU, et al. Spurious Hb mass increases following exercise. Int J Sports Med 2012;33:691-5. [Crossref] [PubMed]
- Lippi G, Schena F, Salvagno GL, et al. Foot-strike haemolysis after a 60-km ultramarathon. Blood Transfus 2012;10:377-83. [PubMed]
- Binnie MJ, Dawson B, Pinnington H, et al. Effect of training surface on acute physiological responses after interval training. J Strength Cond Res 2013;27:1047-56. [Crossref] [PubMed]
- Binnie MJ, Dawson B, Pinnington H, et al. Part 2: effect of training surface on acute physiological responses after sport-specific training. J Strength Cond Res 2013;27:1057-66. [Crossref] [PubMed]
- Christensen DL, Espino D, Infante-Ramirez R, et al. Normalization of elevated cardiac, kidney, and hemolysis plasma markers within 48 h in Mexican Tarahumara runners following a 78 km race at moderate altitude. Am J Hum Biol 2014;26:836-43. [Crossref] [PubMed]
- Robach P, Boisson RC, Vincent L, et al. Hemolysis induced by an extreme mountain ultra-marathon is not associated with a decrease in total red blood cell volume. Scand J Med Sci Sports 2014;24:18-27. [Crossref] [PubMed]
- Chiu YH, Lai JI, Wang SH, et al. Early changes of the anemia phenomenon in male 100-km ultramarathoners. J Chin Med Assoc 2015;78:108-13. [Crossref] [PubMed]
- Caulfield S, McDonald KA, Dawson B, et al. A comparison of haemolytic responses in fore-foot and rear-foot distance runners. J Sports Sci 2016;34:1485-90. [Crossref] [PubMed]
- Liu CH, Tseng YF, Lai JI, et al. The changes of red blood cell viscoelasticity and sports anemia in male 24-hr ultra-marathoners. J Chin Med Assoc 2018;81:475-81. [Crossref] [PubMed]
- Beneke R, Bihn D, Hutler M, et al. Haemolysis caused by alterations of alpha- and beta-spectrin after 10 to 35 min of severe exercise. Eur J Appl Physiol 2005;95:307-12. [Crossref] [PubMed]
- Selby GB, Eichner ER. Endurance swimming, intravascular hemolysis, anemia, and iron depletion. New perspective on athlete's anemia. Am J Med 1986;81:791-4. [Crossref] [PubMed]
- Poortmans JR. Exercise and renal function. Sports Med 1984;1:125-53. [Crossref] [PubMed]
- Rasmussen H, Lake W, Allen JE. The effect of catecholamines and prostaglandins upon human and rat erythrocytes. Biochim Biophys Acta 1975;411:63-73. [Crossref] [PubMed]
- Yamada T, Tohori M, Ashida T, et al. Comparison of effects of vegetable protein diet and animal protein diet on the initiation of anemia during vigorous physical training (sports anemia) in dogs and rats. J Nutr Sci Vitaminol (Tokyo) 1987;33:129-49. [Crossref] [PubMed]
- Richieri GV, Mel HC. Temperature effects on osmotic fragility, and the erythrocyte membrane. Biochim Biophys Acta 1985;813:41-50. [Crossref] [PubMed]
- Hiro T. Studies on the osmotic fragility of erythrocytes influenced by a metabolic acidosis. Jpn J Phys Fitness Sports Med 1982;31:279-90. [Crossref]
- Sikora J, Orlov SN, Furuya K, et al. Hemolysis is a primary ATP-release mechanism in human erythrocytes. Blood 2014;124:2150-7. [Crossref] [PubMed]
- Platt OS, Lux SE, Nathan DG. Exercise-induced hemolysis in xerocytosis. Erythrocyte dehydration and shear sensitivity. J Clin Invest 1981;68:631-8. [Crossref] [PubMed]
- Smith JA, Kolbuch-Braddon M, Gillam I, et al. Changes in the susceptibility of red blood cells to oxidative and osmotic stress following submaximal exercise. Eur J Appl Physiol Occup Physiol 1995;70:427-36. [Crossref] [PubMed]
- Godal HC, Refsum HE. Haemolysis in athletes due to hereditary spherocytosis. Scand J Haematol 1979;22:83-6. [Crossref] [PubMed]
- Connes P, Machado R, Hue O, et al. Exercise limitation, exercise testing and exercise recommendations in sickle cell anemia. Clin Hemorheol Microcirc 2011;49:151-63. [PubMed]
- Eziokwu AS, Angelini D. New Diagnosis of G6PD Deficiency Presenting as Severe Rhabdomyolysis. Cureus 2018;10:e2387. [PubMed]
- Kim J, Borges WH, Holliday MA. Correlation between RBC osmotic fragility and serum sodium. Am J Dis Child 1962;104:281-8. [PubMed]
- Lippi G, Mercadanti M, Aloe R, et al. Erythrocyte mechanical fragility is increased in patients with type 2 diabetes. Eur J Intern Med 2012;23:150-3. [Crossref] [PubMed]
- Horii K, Adachi Y, Ohba Y, et al. Erythrocyte osmotic fragility in various liver diseases--application of coil planet centrifuge system. Gastroenterol Jpn 1981;16:161-7. [Crossref] [PubMed]
- Schumacher YO, Jankovits R, Bultermann D, et al. Hematological indices in elite cyclists. Scand J Med Sci Sports 2002;12:301-8. [Crossref] [PubMed]
- Lupon J, Urrutia A, Gonzalez B, et al. Prognostic significance of hemoglobin levels in patients with heart failure. Rev Esp Cardiol 2005;58:48-53. [PubMed]
- Rother RP, Bell L, Hillmen P, et al. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA 2005;293:1653-62. [Crossref] [PubMed]
- Schaer CA, Deuel JW, Bittermann AG, et al. Mechanisms of haptoglobin protection against hemoglobin peroxidation triggered endothelial damage. Cell Death Differ 2013;20:1569-79. [Crossref] [PubMed]
- Kokkinos P. Physical activity, health benefits, and mortality risk. ISRN Cardiol 2012;2012:718789. [Crossref] [PubMed]
- Liu CK, Fielding RA. Exercise as an intervention for frailty. Clin Geriatr Med 2011;27:101-10. [Crossref] [PubMed]
- van Eck CF, Fu FH. The benefits of youth sports participation should outweigh the risks. Ann Transl Med 2018;6:S11. [Crossref] [PubMed]