
Adjacent level fracture incidence in single fraction high dose spinal radiosurgery
Introduction
Spinal metastases occur in approximately 20,000 American patients each year (1,2). With advances in chemotherapy, biologic therapy, immunotherapy, surgical techniques and radiation treatment, patients are living longer with better quality of life, and the number of patients experiencing spinal metastatic disease is anticipated to grow further. Spine stereotactic radiosurgery (SRS) has emerged as a method to provide local control with minimal toxicity or interruption in systemic therapy. SRS provides highly conformal radiation to tumor volumes with steep radiation gradients to avoid injury to local structures such as the spinal cord and esophagus. Treatment of spinal metastases is palliative, but safe and excellent durable local disease control has been achieved with local control rates of 70–90% or higher at 1 year or longer with the use of new treatment paradigms (3-7). Despite impressive local control, SRS has complications, the most studied of which is vertebral compression fractures (VCFs).
VCFs following radiosurgery at the irradiated level have been reported at incidences ranging from 6–39% (8-14). The vertebral body level, amount of vertebral body replaced with tumor (10), patient age, pre-existing fracture (8) and radiation dose (9,11,14) have all been reported as risk factors for VCF. Thus far, studies have evaluated the incidence and risk factors for VCF at the level of vertebral tumor invasion and not for VCF occurring in vertebral bodies adjacent to the target treatment level. However, VCFs above and below the irradiated level have occurred. This paper represents the first study to our knowledge evaluating adjacent level VCFs in patients undergoing spinal SRS.
Methods
IRB approval was obtained. We identified 206 patients who underwent single fraction radiosurgery in a single dose of 24 Gy to the mobile spine from 2011 to 2014 from a prospective database. Patients with sacral SRS were excluded. Patients undergoing multiple single-fraction SRS treatments were included so long as therapy was directed at non-adjacent vertebral levels. A total of 239 treatments met inclusion criteria. All patients had a histologically confirmed diagnosis of malignancy. Patients underwent simulation for radiation planning with CT images with 2-mm slice thickness. A myelogram or magnetic resonance image fusion was utilized to delineate spinal cord anatomy and tumor volumes. Patients were immobilized using a patient-customized cradle for both SRS simulation and therapy (6). Treatment planning was performed with either in-house software or Eclipse (Varian Medical Systems) with inverse treatment planning. The gross tumor volume (GTV) was outlined according to CT and MRI images after review by the treating radiation oncologist and neurosurgeon. The clinical target volume (CTV) encompassed GTV as well as adjacent bone according to Cox et al. (15). The planning target volume (PTV) was a 2 mm expansion from CTV, excluding thecal sac and also esophagus if not abutting GTV. The prescribed dose was 24 Gy to the PTV in a single fraction, and dose was prescribed to the 100% isodose line as allowed by spinal cord dose constraints. Dose constraints were defined per the standard at our institution. The spinal cord, defined on a simulation CT myelogram, was constrained to a maximum dose of 14 Gy to a single voxel, or 12 Gy if circumferential PTV or prior radiation treatment. The cauda equina and brainstem were limited to maximum dose of 18 Gy. The esophagus was constrained to 14 Gy to 2.5 mL of esophagus. Bowel was constrained to allow no more than 5 mL to receive more than 16 Gy.
Treatment was delivered with linear accelerators using 6-mv and/or 15-mv photons. Cone-beam CTs were utilized to verify patient positioning prior to treatment. A representative treatment plan is shown in Figure 1.
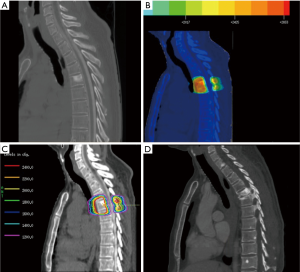
A retrospective chart review was carried out collecting patient and tumor characteristics and follow-up information. The primary outcome was development of an adjacent level VCF (which included endplate fractures and collapse deformities). Each patient had at least one documented imaging methodology for review. For each patient included, all pre-SRS treatment and post-SRS imaging (CT, MRI, plain films) radiology reports were initially screened for mention of adjacent level fractures or similar indication. Vertebral body fractures were defined as loss of vertebral body height or endplate infarctions, adjacent to the level of prior radiation. Positive findings were documented, and all associated images underwent subsequent viewing to confirm fracture state. After further viewing, images that remained under question underwent further review by a single neuroradiologist for final diagnosis. Cox regression univariate analysis modeling was performed with IBM SPSS software to determine any associations between clinical factors and adjacent level VCFs.
The fractured endplates of all pure adjacent segment VCFs were contoured as well as sixteen non-fractured endplates, and dose volume histograms were calculated. Mean and maximum doses in Gy were collected.
Results
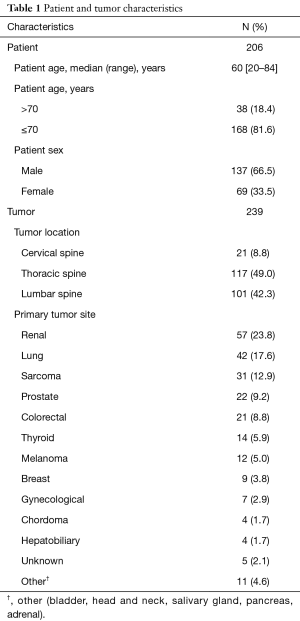
Patient and tumor characteristics are summarized in Table 1. There were 206 patients treated to a total of 239 treatment sites with SRS to the mobile spine, and all patients were treated to dose 24 Gy in 1 fraction. The median patient age was 60 years (range, 20–84 years), and 18.6% patients were greater than age 70. The majority of patients were male (66.5%). A total of 198 patients (96.1%) underwent SRS for metastatic disease to the spine, while 8 patients (3.9%) were treated for primary spinal tumors. The most common tumor histology was carcinoma (78%), as compared to sarcoma or other. The majority of tumors treated were located in the thoracic spine (49.0%) or lumbar spine (42.3%), followed by cervical spine (8.8%). The median follow-up was 14.4 months (range, 0–64 months) from date of SRS. Eighteen patients, comprising 20 treated lesions, had no post-SRS imaging for review.
Full table
There were 26 incidents (10.8% of treatments) of adjacent level VCFs observed in 26 patients. Five cases had both instrumentation and metastatic disease present at the level of VCF. Fourteen cases occurred at sites harboring metastatic disease without hardware present. There were seven (2.9% of all treatments) adjacent level VCFs without hardware or metastases present at the level of VCF. Five of these seven cases had compression fracture of the irradiated index level as well. The median time to fracture post-SRS was 13.5 months (range, 2.1–35.1 months) for all adjacent level VCFs. Asymptomatic fractures were monitored with follow-up imaging. Symptomatic patients were treated using pain medications and, if the pain did not resolve, underwent cement stabilization of the fracture.
In a subset analysis of the seven adjacent level VCFs without metastases or hardware, the median time to adjacent level VCF was the same at 13.5 months post-SRS (range, 5–25 months). There were two lumbar, three thoracic and two cervical pure adjacent VCFs. There were three mobile spine, two junctional, and two semi-rigid locations according to spinal instability neoplastic score (SINS) criteria (16). Five of the seven (71%) pure adjacent level VCFs were associated with collapse of the irradiated vertebral body, three occurring simultaneously and two afterward. All pure adjacent level VCFs occurred after SRS. Four of the adjacent level fractures occurred at the endplate adjacent to the irradiated vertebral body, and three adjacent level fractures occurred at the opposite endplate. The mean of the mean dose to adjacent level fractured endplate immediately adjacent to the irradiated vertebral body was 22.5 Gy, and the median mean dose was 23.3 Gy. The mean of the mean dose of sixteen non-fractured endplates immediately adjacent to the SRS site was 18.8 Gy with a median mean dose of 19.1 Gy.
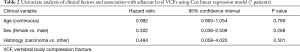
Cox regression analysis was performed for the seven adjacent level fractures without hardware or metastatic disease with a univariate model for sex, age, and tumor histology for VCF. No statistically significant differences were noted, as shown in Table 2.
Full table
This was again performed for the subset of four patients who had fractures at endplates adjacent to the irradiated vertebral body and is shown in Table 3.

Full table
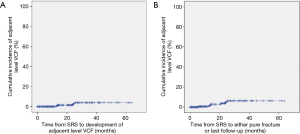
A cumulative incidence curve for the development of adjacent level VCF is shown in Figure 2.
Conclusions
To our knowledge, these data represent the first study evaluating adjacent level VCFs in spine SRS. Given that spine SRS is palliative in nature, avoiding complications is desirable. The number of patients receiving spinal SRS will continue to increase and therefore knowledge of its complications are important for both patients and physicians. Previous studies have demonstrated a radiation dose dependent risk of fracture in the axial skeleton in both traditionally fractionated and hypofractionated treatment schedules (17,18). While the intent of radiosurgery is to treat the tumor volume along with tissues at risk of microscopic tumor invasion, adjacent organs at risk (OAR) receive subtherapeutic radiation doses. While VCF at target site has been studied, presence of fracture at adjacent-level endplates has not been reported. As demonstrated in Figure 1, adjacent level endplates can receive doses at or near 20 Gy. Interestingly, in this study, the average radiation to the fractured adjacent level endplate was 23.3 Gy. It should be noted that conformality of SRS dose is more precise in the axial plane than the sagittal plane, and that some excess dose may be delivered to the adjacent endplates as part of this error arising from uncertainty in patient positioning, machine calibration, imaging, etc. (19). Endplates of adjacent levels may experience higher doses than planned, contributing to adjacent level collapse. In our study, multilevel vertebral body compression fractures were present in 71.4% of the adjacent level fractures indicating some poorly understood disruption of local biomechanics may be implicated in adjacent level fractures as well.
This paper serves to define the incidence of adjacent level fractures in those undergoing spinal SRS and raises the question of whether adjacent level endplates should be considered an OAR during SRS planning, similar to the spinal cord, esophagus, or kidneys.
In conclusion, we report that adjacent level VCFs are infrequent in the setting of single fraction SRS to the mobile spine, occurring in 2.9% of treatments. Understanding the incidence is important for care in patients undergoing spinal SRS, as use of SRS to treat spinal pathologies will continue to increase in number. Further studies are needed to validate these results and determine risk factors to adjacent level VCF in an effort to avoid or minimize this complication. Given our findings, further investigation is warranted as to whether adjacent level endplates should be considered an organ at risk during SRS planning.
Acknowledgments
Funding: This work was supported in part by the MSKCC Cancer Center Support Grant (NIH/NCI P30 CA008748).
Footnote
Conflicts of Interest: Dr. Yamada is a consultant for Varian Medical Systems and on the Speakers’ Bureau of the Institute for Medical Education. Dr. Laufer receives consulting fees from DePuy/Synthes, SpineWave and Globus. Dr. Bilsky receives consulting fees from DePuy/Synthes, Globus and BrainLab. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by IRB ethics committee (No. 16-1434).
References
- Ecker RD, Endo T, Wetjen NM, et al. Diagnosis and treatment of vertebral column metastases. Mayo Clin Proc 2005;80:1177-86. [Crossref] [PubMed]
- Schiff D. Spinal cord compression. Neurol Clin 2003;21:67-86. viii. [Crossref] [PubMed]
- Gerszten PC, Burton SA, Ozhasoglu C, et al. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976) 2007;32:193-9. [Crossref] [PubMed]
- Laufer I, Iorgulescu JB, Chapman T, et al. Local disease control for spinal metastases following "separation surgery" and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: outcome analysis in 186 patients. J Neurosurg Spine 2013;18:207-14. [Crossref] [PubMed]
- Moussazadeh N, Laufer I, Yamada Y, et al. Separation surgery for spinal metastases: effect of spinal radiosurgery on surgical treatment goals. Cancer Control 2014;21:168-74. [Crossref] [PubMed]
- Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys 2008;71:484-90. [Crossref] [PubMed]
- Al-Omair A, Smith R, Kiehl TR, et al. Radiation-induced vertebral compression fracture following spine stereotactic radiosurgery: clinicopathological correlation. J Neurosurg Spine 2013;18:430-5. [Crossref] [PubMed]
- Boehling NS, Grosshans DR, Allen PK, et al. Vertebral compression fracture risk after stereotactic body radiotherapy for spinal metastases. J Neurosurg Spine 2012;16:379-86. [Crossref] [PubMed]
- Cunha MV, Al-Omair A, Atenafu EG, et al. Vertebral compression fracture (VCF) after spine stereotactic body radiation therapy (SBRT): analysis of predictive factors. Int J Radiat Oncol Biol Phys 2012;84:e343-9. [Crossref] [PubMed]
- Rose PS, Laufer I, Boland PJ, et al. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J Clin Oncol 2009;27:5075-9. [Crossref] [PubMed]
- Sahgal A, Atenafu EG, Chao S, et al. Vertebral compression fracture after spine stereotactic body radiotherapy: a multi-institutional analysis with a focus on radiation dose and the spinal instability neoplastic score. J Clin Oncol 2013;31:3426-31. [Crossref] [PubMed]
- Moussazadeh N, Lis E, Katsoulakis E, et al. Five-Year Outcomes of High-Dose Single-Fraction Spinal Stereotactic Radiosurgery. Int J Radiat Oncol Biol Phys 2015;93:361-7. [Crossref] [PubMed]
- Germano IM, Carai A, Pawha P, et al. Clinical outcome of vertebral compression fracture after single fraction spine radiosurgery for spinal metastases. Clin Exp Metastasis 2016;33:143-9. [Crossref] [PubMed]
- Jawad MS, Fahim DK, Gerszten PC, et al. Vertebral compression fractures after stereotactic body radiation therapy: a large, multi-institutional, multinational evaluation. J Neurosurg Spine 2016;24:928-36. [Crossref] [PubMed]
- Cox BW, Spratt DE, Lovelock M, et al. International Spine Radiosurgery Consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2012;83:e597-605. [Crossref] [PubMed]
- Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976) 2010;35:E1221-9. [Crossref] [PubMed]
- Overgaard M. Spontaneous radiation-induced rib fractures in breast cancer patients treated with postmastectomy irradiation. A clinical radiobiological analysis of the influence of fraction size and dose-response relationships on late bone damage. Acta Oncol 1988;27:117-22. [Crossref] [PubMed]
- Pettersson N, Nyman J, Johansson KA. Radiation-induced rib fractures after hypofractionated stereotactic body radiation therapy of non-small cell lung cancer: a dose- and volume-response analysis. Radiother Oncol 2009;91:360-8. [Crossref] [PubMed]
- Mack A, Mack G, Weltz D, et al. Quality assurance in stereotactic space. Determination of the accuracy of aim and dose in single dose radiosurgery. Strahlenther Onkol 2003;179:760-6. [Crossref] [PubMed]


