
A retrospective study of the tolerability of nintedanib for severe idiopathic pulmonary fibrosis in the real world
Introduction
Idiopathic pulmonary fibrosis (IPF), a pathologically usual interstitial pneumonia (UIP), is the most common form of idiopathic interstitial pneumonia (IIP) (1-3). Estimates of the IPF prevalence per 100,000 people were reported as ranging from 2 to 43 cases globally, with 10 cases in Japan (1,4). Patients with IPF have a grave prognosis with a median survival of 2–5 years (1-3), the major cause of death being acute exacerbation (AE) and/or gradual respiratory failure (4). It has been hypothesized that the main pathogenesis of IPF progression is aberrant wound healing and fibrosis to initial epithelial injury (5). Nintedanib is a triple kinase inhibitor targeting the platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) receptors, which are molecules that contribute to over repairing. The INPULSIS®-1 and -2 trials, conducted as above two replicate phase 3 trials, demonstrated that nintedanib significantly reduced the decline of forced vital capacity (FVC) during 52 weeks in IPF patients (nintedanib groups; −2.8% and −3.1% predicted, placebo groups; −6.0% and −6.2% predicted in INPULSIS®-1 and -2 trials, respectively) (6). The most frequent adverse event of nintedanib was diarrhea with rates of 62.4% in INPULSIS® trials (6,7). However, the proportion of patients who discontinued taking nintedanib caused due to diarrhea was less than 5%. Although elevation of serum aminotransferase levels to more than three times the upper limit of the normal range was seen in 5.0% of the patients in the INPULSIS® trials, these elevations were reversible (6,7). Brunnemer et al. suggested that diarrhea was the most common nintedanib-induced adverse event (33%) but was generally manageable by temporary discontinuation and dose reduction in the real world (8). These results suggested that nintedanib has a high efficacy and a manageable tolerability in IPF patients.
The results of the INPULSIS® trials suggested that there was no significant difference in the decline of FVC during 52 weeks between patients with baseline FVC values of more than 70% and 50–70% predicted (6). However, the eligibility criteria for patient inclusion in the INPULSIS® trials included FVC of >50% predicted and diffusing capacity of the lung for carbon monoxide (DLCO) of 30–79% predicted. The INPULSIS® trials did not clarify the efficacy of nintedanib for severe IPF patients with low pulmonary function. Wuyts et al. analyzed the efficacy of nintedanib for patients with severe IPF who had an FVC of ≤ 50% predicted and entered an open label extension of the INPULSIS® trials (INPULSIS®-ON trial). That study suggested that there was no significant difference of FVC decline in the INPULSIS®-ON trial between patients who had a baseline FVC values of ≤ 50% predicted and those with values of >50% predicted (decline in FVC; −62.3 and −87.9 mL, number; 24 and 558, respectively) (9). However, the available data to clarify the tolerability of nintedanib for severe IPF patients is limited in clinical practice. In this retrospective study, we analyzed whether nintedanib was tolerable for severe IPF patients who would not have been eligible for entry into the INPULSIS® and INPULSIS-On® trials due to low pulmonary function.
Methods
Study subjects
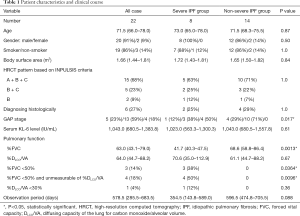
The characteristics of the study subjects are shown in Table 1. We retrospectively reviewed medical records and high-resolution computed tomography (HRCT) images covering the period between July 2015 and August 2016 for all subjects. We included 22 patients (20 males, median age 71.5 years) treated with nintedanib at either our hospital or an affiliated one. All included patients were diagnosed as having IPF in accordance with the recent official guidelines (1-3). Other diseases such as connective tissue diseases were excluded. Patients complicated by malignant disease who had not undergone radical therapy were also excluded, as such patients show poor survival and deterioration of their general condition, making it difficult to evaluate the therapeutic effect of nintedanib for IPF.
Full table
The criteria we used to define “severe IPF” group were FVC <50% predicted, DLCO/alveolar volume (DLCO/VA) <30% predicted, or unmeasurable DLCO/VA due to having low FVC. These criteria overlapped with some of the exclusion criteria for the INPULSIS® trials (6). Patients with IPF who met none of the criteria for severe IPF were classified into a “non-severe IPF” group. The disease severity staging was performed in accordance with the GAP score as reported previously i.e., classifying patients into three stages based on clinical (e.g., gender, age) and physiologic (e.g., FVC, DLCO) variables (10). The GAP stage was able to predict 1-, 2-, and 3-year mortalities for IPF (10).
We received specific approval for all procedures from the Institutional Review Board (IRB) of our hospital in accordance with the ethical standards of the Helsinki Declaration of 2013. The IRB approval No. and date of approval for our study were 16186 and 30 November 2016, respectively.
Interpretation of high-resolution computed tomography images
HRCT examinations without contrast medium were performed at the start of treatment with nintedanib using a variety of scanners. The protocol consisted of end-inspiration in the supine position, and 0.5- to 1.5-mm collimation sections reconstructed with a high-spatial-frequency algorithm at 1- or 2-cm intervals. Images were interpreted at a window setting appropriate for viewing the lung parenchyma [window level, −600 to −700 Hounsfield units (HU); window width, 1,200 to 1,500 HU].
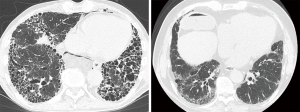
We classified IPF patients by HRCT in accordance with the radiological criteria used for the INPULSIS® trials (6). Briefly, if the HRCT findings for a subject corresponded to A, B and C as described below, A and C, or B and C, we considered them to be consistent with a UIP pattern. These INPULSIS criteria based on HRCT were as follows: (A) definite honeycomb lung destruction with basal and peripheral predominance; (B) presence of reticular abnormality and traction bronchiectasis consistent with fibrosis showing basal and peripheral predominance; (C) atypical features are absent, specifically: nodules and consolidation. Ground-glass attenuation (GGA), if present, is less extensive than a reticular opacity pattern. Two board-certificated diagnostic radiologists, who specialized in diffuse lung diseases and had 31 and 10 years of experience in chest CT interpretation, independently evaluated the HRCT findings and categorized each case into the INPULSIS criteria A, B, or C. The radiologists were blinded to the clinical information. Representative HRCT images are shown in Figure 1. Disagreements about the HRCT findings between the two radiologists were resolved by consensus after assessing the inter-observer agreement.
Evaluation of treatment tolerability and efficacy
We evaluated the severity of adverse events using the Common terminology criteria for adverse events version 4.0 (CTCAE ver. 4.0) described as supplementary materials. We analyzed differences in the incidence and CTCAE ver. 4.0 grade of nintedanib-related adverse event and in the incidence of permanent or temporary discontinuation of nintedanib due to adverse event.
For evaluating the treatment efficacy, we analyzed changes in FVC and serum KL-6 levels, which is reported as a diagnostic and prognostic biomarker of IPF (11-13), over 6 months.
Statistical analysis
Data were expressed as the median [25th to 75th percentiles of the interquartile range (IQR)]. Differences between two groups were analyzed appropriately by the Wilcoxon-rank sum test or Fisher’s exact test. P values <0.05 was taken to represent statistical significance. All statistical analyses were performed using the JMP 13.0 program (SAS Institute Japan, Tokyo, Japan).
Results
Inter-observer agreement of HRCT image interpretation
There was significant agreement between the 2 observers with regard to their assessment of individual cases as to whether the HRCT findings met the INPULSIS criteria A, B, or C (kappa =0.56, P<0.01). After consensus reading of the HRCT images, 15 cases (68%) were classified as having criteria A, B and C, 5 (23%) as having B and C, and 2 (9%) as having B alone. Twenty cases who met criteria A, B, and C or B and C were considered to be consistent with UIP pattern. The remaining 2 cases who met only criteria B were inconsistent with UIP pattern and diagnosed as IPF based on histological features of lung tissue obtained by surgical lung biopsy (SLB).
Patient characteristics and clinical course
Table 1 shows patient characteristics and clinical course and Table 2 summarized the individual characteristics of the patients with severe IPF. One patient with IPF and stage 4 non-small cell lung cancer was excluded from this study. Six patients were able to be diagnosed as UIP, histologically. Among them, 3 patients underwent SLB for differential diagnosis of chronic hypersensitivity pneumonia or smoking-related interstitial pneumonia and 3 patients underwent lobectomy for lung cancer. Median FVC for all subjects was 63.0% (43.1–79.0%) predicted and median DLCO/VA was 64.0% (44.7–88.2%) predicted at the baseline. Eight (36%) of the patients were classified as having severe IPF and 14 (64%) as having non-severe IPF. Of the 8 subjects in the severe IPF group, 3 patients (14%) had FVC of <50% predicted with DLCO/VA of ≥30% predicted, 4 patients (18%) had FVC of <50% predicted with unmeasurable DLCO/VA, and 1 patient (4%) had DLCO/VA of <30% predicted with FVC of ≥50% predicted. The baseline FVC was lower in the severe IPF group than in the non-severe IPF group (P=0.0013). The disease severity staging based on the GAP score was higher in the severe IPF group than in the non-severe IPF group (P=0.017). Four (18%) of the 22 patients received drug therapies in combination with nintedanib at the start of therapy. Those therapies included corticosteroid alone in 2 subjects and corticosteroid with cyclosporine A in 2. The overall median treatment period was 578.5 (285.5–683.5) (range: 47.0–787.0) days, and this period did not differ between the severe and non-severe IPF groups.

Full table
Evaluation of tolerability
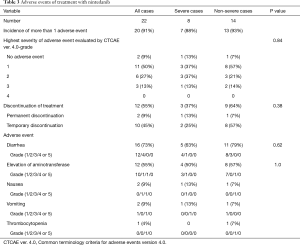
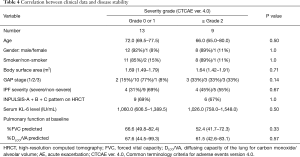
Table 3 gives details of the adverse events. A total of 33 drug-related adverse events were observed in 20 (91%) of the 22 patients with IPF. The highest severity of adverse events evaluated by CTCAE ver. 4.0 was grade 1 in 11 patients (50%), grade 2 in 6 (27%), and grade 3 in 3 (13%). None of the patient had grade 4 or 5 adverse event. Specific adverse events included diarrhea in 16 patients (73%), elevation of the serum aspartate or alanine aminotransferase level in 12 (55%), nausea in 2 (9%), vomiting in 2 (9%), and thrombocytopenia in 1 (4%). Only 2 (9%) of the 22 patients, including 1 each with severe and non-severe IPF, required permanent discontinuation of nintedanib due to adverse events. Temporary discontinuation of nintedanib was necessary in 10 (45%) of the 22 patients, including 2 with severe and 8 with non-severe IPF. The adverse events that led to permanent discontinuation of nintedanib included vomiting in 1 patients and nausea with thrombocytopenia in 1. All IPF patients were started on 300 mg/day nintedanib. Fifteen (68%) of the 22 patients, including 4 with severe and 11 with non-severe IPF, required a reduction in the nintedanib dose to 200 mg/day due to adverse events. There was no difference in the incidence of total adverse events, diarrhea or elevation of the serum aminotransferase level as specific adverse event, or in the incidence of permanent or temporary discontinuation of nintedanib in between severe and non-severe IPF groups. No clinical data, including IPF severity, was associated with the proportion of patients who had an adverse event with CTCAE grade ≥2 (Table 4) or the incidence of permanent or temporary discontinuation of nintedanib (data not shown). These results suggest that the baseline severity of IPF was not associated with nintedanib tolerability.
Full table
Full table
Evaluation of efficacy of nintedanib
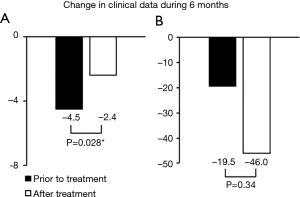
Changes in FVC and serum KL-6 level during 6 months prior to and after treatment are shown in Figure 2. Changes in FVC both prior to and after treatment were unmeasurable in 2 of severe IPF patients due to deterioration of IPF or death. In addition, change in FVC after treatment was unmeasurable in 1 severe IPF patients. Changes in FVC prior to and after treatment were measurable for all non-severe IPF patients, and changes in serum KL-6 levels were measurable in all subjects. For the total group of subjects, declines in FVC after treatment were significantly lower than those prior to treatment (median FVC change: −2.4% and −4.5% predicted; subject number: 20 and 19, respectively; P=0.028). In contrast, there was no difference in the change in serum KL-6 level between the 6 months prior to and after the nintedanib treatment (median values; −19.5 and −46.0 IU/mL, number; both of 22, P=0.34).
Discussion
In the present study, the major adverse events of nintedanib were the diarrhea and elevation of serum aminotransferase level. These adverse events were mild to moderate in severity and did not lead to permanent discontinuation of nintedanib. Among the 22 IPF patients, nausea, vomiting or thrombocytopenia led to permanent discontinuation of nintedanib in only 2 patients, of whom 1 had severe IPF. These results indicate that nintedanib treatment for IPF patients was well tolerated, as reported in the INPULSIS® trials and the previous real world study (6,8).
We classified the 22 patients with IPF into severe and non-severe cases on the basis of baseline FVC and DLCO. Some previous reports have suggested that low FVC and/or DLCO at the baseline are associated with poor survival in IPF patients (4,14-18). In the present study, 8 patients with severe IPF have a higher disease severity staging based on the GAP index than those with non-severe IPF. We discovered that there was no difference in the incidence or severity of adverse events or in the proportion of permanent or temporary nintedanib discontinuation between the groups with severe and non-severe IPF. Wuyts et al. suggested that incidence of diarrhea led to treatment discontinuation were similar between patients with baseline FVC ≤50% and >50% predicted, respectively (4.9% and 5.4%), as reported in the INPULSIS-On® trials (9). Recently, the INSTAGE® trial was conducted to evaluate the efficacy and safety of combination therapy with nintedanib and sildenafil as compared with nintedanib alone for patients with severe IPF with DLCO of <35% predicted. In this trial, the proportions of patients who had a reduction in dose, or interruption or permanent discontinuation of nintedanib due to adverse events, were similar to those in the INPULSIS® trials (19). However, randomized controlled trials are unable to accurately replicate the clinical practice, because the subjects selected may be younger or have fewer comorbidities. In the present study, we suggested that nintedanib was tolerable for severe IPF patients who would not have been eligible for entry into the INPULSIS® and INPULSIS-On® trials due to low pulmonary function. It is believed that patients in poor general condition have a higher risk of drug-related adverse events. Indeed, it has been reported that poor performance status is a risk factor for epidermal growth factor receptor tyrosine kinase inhibitor-related ILD (20). Clinicians are likely concerned that initiation of nintedanib treatment for advanced IPF may increase the risk of adverse events. On the other hand, anti-fibrotic drug therapy is started for more than a few cases with severe IPF in Japan, because severe patients can receive public medical expenses (21). A Careful consideration is required to decide whether to perform anti-fibrotic drug treatment or palliative therapy only for severe IPF patients. Our study results suggest that initiation of nintedanib treatment should not be dismissed solely for reasons of low pulmonary function.
In the present study, no clinical parameters at the baseline were associated with nintedanib treatment tolerability. Although Ikeda et al. had suggested that a low body surface area was associated with hepatotoxicity of nintedanib with CTCAE ver.4.0 grade ≥2, this association was not confirmed in the present study (22). However, the discrepancy may be caused by the difference in the incidence of elevated aminotransferase with CTCAE ver.4.0 grade ≥2 between our study and theirs (9% vs. 23.5%, respectively).
In the present study, the significant reduction of decline in FVC prior to and after treatment for the subjects overall may be associated with the suppressive effect of nintedanib on FVC decline that was reported in the INPULSIS® trials. Our study was unable to clarify the efficacy of nintedanib for severe IPF patients, because change in FVC prior to and after treatment was unable to be measured in all patients with severe IPF. However, the previous study suggested that nintedanib was effective for the severe IPF patients who had FVC of ≤50% predicted and entered the INPULSIS®-ON trial (9). This result suggests that nintedanib treatment may be effective for severe IPF patients even in clinical practice. A prospective study is needed to clarify the efficacy of nintedanib for severe IPF in the real world.
The present study had some limitations. First, it was the retrospective single-center study involving a small number of patients with severe IPF. However, we consider that our findings regarding the tolerability of nintedanib nevertheless have clinical importance. Second, we evaluated only the short-term efficacy of nintedanib treatment. We intend to analyze the efficacy and tolerability of nintedanib for a longer period in a further study with larger sample size. Third, change of FVC could not be measured in all subjects. This may have affected our results.
Conclusions
We suggested that nintedanib was tolerable for severe IPF patients who would not have been eligible for entry into the previous clinical trials due to low pulmonary function. Although the therapeutic strategy for severe IPF should be planned carefully, the initiation of nintedanib treatment should not be dismissed solely for reasons of low pulmonary function.
Supplementary
Patients and methods
Evaluation of tolerability
We evaluated the severity of adverse events using Common terminology criteria for adverse events, version 4.0 (CTCAE ver. 4.0). The severity of an elevation in aspartate or alanine transaminase (AST or ALT, respectively) was defined as follows: grade 1 was >3.0× the upper limit of the normal range (IU/L), grade 2 was >3.0–5.0× the upper limit of the normal range, grade 3 was >5.0–20.0× the upper limit of the normal range, and grade 4 was >20.0× the upper limit of the normal range. Diarrhea severity was defined as follows: grade 1 was an increase of <4 stools per day over baseline or a mild increase in ostomy output compared with baseline; grade 2 was increase of 4–6 stools per day over baseline or a moderate increase in ostomy output compared with baseline; grade 3 was an increase of ≥7 stools per day over baseline, incontinence, hospitalization, severe increase in ostomy output compared with baseline, or omitting self-care activities of daily living; and grade 4 was life-threatening consequences or urgent intervention indicated. Nausea severity was defined as follows: grade 1 was loss of appetite without alteration in eating habits; grade 2 was oral intake decreased without significant weight loss, dehydration, or malnutrition; and grade 3 was inadequate oral caloric or fluid intake, tube feeding, total parenteral nutrition (TPN), or hospitalization. Vomiting severity was defined as follows: grade 1 was 1–2 episodes (separated by 5 min) in 24 h; grade 2 was 3–5 episodes (separated by 5 min) in 24 h; grade 3 was ≥6 episodes (separated by 5 min) in 24 h, tube feeding, TPN, or hospitalization; and grade 4 was life-threatening consequences or urgent intervention indicated. Thrombocytopenia severity was defined as follows: grade 1 was platelet count decreased below the lower limit of normal to 7.5×104 platelets/µL, grade 2 was <7.5–5.0×104 platelets/µL, and grade 3 was <5.0 to <2.5×104 platelets/µL.
Acknowledgments
We would like to thank past and present doctors and technicians in our laboratory.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board (IRB) of our hospital (No. 16186).
References
- Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:e44-68. [Crossref] [PubMed]
- Raghu G, Rochwerg B, Zhang Y, et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med 2015;192:e3-19. [Crossref] [PubMed]
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- Natsuizaka M, Chiba H, Kuronuma K, et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med 2014;190:773-9. [Crossref] [PubMed]
- Strieter RM, Mehrad B. New mechanisms of pulmonary fibrosis. Chest 2009;136:1364-70. [Crossref] [PubMed]
- Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071-82. [Crossref] [PubMed]
- Corte T, Bonella F, Crestani B, et al. Safety, tolerability and appropriate use of nintedanib in idiopathic pulmonary fibrosis. Respir Res 2015;16:116. [Crossref] [PubMed]
- Brunnemer E, Wälscher J, Tenenbaum S, et al. Real-World Experience with Nintedanib in Patients with Idiopathic Pulmonary Fibrosis. Respiration 2018;95:301-9. [Crossref] [PubMed]
- Wuyts WA, Kolb M, Stowasser S, et al. First data on efficacy and safety of nintedanib in patients with idiopathic pulmonary fibrosis and forced vital capacity of ≤50% of predicted value. Lung 2016;194:739-43. [Crossref] [PubMed]
- Ryerson CJ, Vittinghoff E, Ley B, et al. Predicting survival across chronic interstitial lung disease: the ILD-GAP model. Chest 2014;145:723-8. [Crossref] [PubMed]
- Kohno N, Awaya Y, Oyama T, et al. KL-6, a mucin-like glycoprotein, in bronchoalveolar lavage fluid from patients with interstitial lung disease. Am Rev Respir Dis 1993;148:637-42. [Crossref] [PubMed]
- Yokoyama A, Kohno N, Hamada H, et al. Circulating KL-6 predicts the outcome of rapidly progressive idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998;158:1680-4. [Crossref] [PubMed]
- Ishikawa N, Hattori N, Yokoyama A, et al. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Investig 2012;50:3-13. [Crossref] [PubMed]
- du Bois RM, Weycker D, Albera C, et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med 2011;184:1382-9. [Crossref] [PubMed]
- King TE Jr, Safrin S, Starko KM, et al. Analyses of efficacy end points in a controlled trial of interferon-gamma1b for idiopathic pulmonary fibrosis. Chest 2005;127:171-7. [Crossref] [PubMed]
- Egan JJ, Martinez FJ, Wells AU, et al. Lung function estimates in idiopathic pulmonary fibrosis: the potential for a simple classification. Thorax 2005;60:270-3. [Crossref] [PubMed]
- Latsi PI, du Bois RM, Nicholson AG, et al. Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. Am J Respir Crit Care Med 2003;168:531-7. [Crossref] [PubMed]
- Mogulkoc N, Brutsche MH, Bishop PW, et al. Pulmonary function in idiopathic pulmonary fibrosis and referral for lung transplantation. Am J Respir Crit Care Med 2001;164:103-8. [Crossref] [PubMed]
- Kolb M, Raghu G, Wells AU, et al. Nintedanib plus Sildenafil in Patients with Idiopathic Pulmonary Fibrosis. N Engl J Med. 2018;379:1722-31. [Crossref] [PubMed]
- Kudoh S, Kato H, Nishiwaki Y, et al. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med 2008;177:1348-57. [Crossref] [PubMed]
- Okuda R, Hagiwara E, Baba T, et al. Safety and efficacy of pirfenidone in idiopathic pulmonary fibrosis in clinical practice. Respir Med 2013;107:1431-7. [Crossref] [PubMed]
- Ikeda S, Sekine A, Baba T, et al. Low body surface area predicts hepatotoxicity of nintedanib in patients with idiopathic pulmonary fibrosis. Sci Rep 2017;7:10811. [Crossref] [PubMed]


