
Pretransplant renal function evaluated by serum cystatin C was associated with mortality after liver transplantation: a single-center experience
Introduction
Liver transplantation (LT) has become the mainstream and only radical therapy for patients with end-stage liver disease (1). Due to the worldwide shortage of liver donors, only a few patients received transplantation in time, and the mortality rate among patients on the waiting list has increased. Therefore, a more reliable pretransplant recipient evaluation system was needed in order to establish a more justifiable allocation system. For achieving such a goal, the model for end-stage liver disease (MELD) score was proposed (2). Compared with the Child-Pugh grading score, MELD score includes renal function, which has been well-established to be associated with the prognosis of liver recipients, as a predictor for patient mortality (3).
In the MELD score, the renal function was reflected by creatinine, which was commonly used for calculating the estimated glomerular filtration rate (eGFR). However, Gonwa et al. (4) stated that creatinine-based equations of eGFR, including Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease 4 (MDRD-4) were neither accurate in the pre- nor post-liver transplant population, especially in patients with advanced liver disease, high bilirubin, or refractory ascites. Thus, a substitutive indicator for renal function in such patients was needed.
Serum cystatin C (CystC) was considered as a more consistent and appropriate alternative endogenous filtration marker to furnish more accurate assessments of eGFR than creatinine. The measurement of CystC was not interfered with by muscle mass, age, gender, jaundice, or hemolysis (5). Also, equations based on CystC, including CKD-EPI-CystC and Hoek formulas, were revealed to have lower bias and higher accuracies than creatinine-based ones in a patient with cirrhosis (5).
In the present study, we aimed to assess the association between eGFR estimated by CKD-EPI-CystC equation and post-transplantation mortality.
Methods
Patients and methods
Patients with simultaneous multiple organ transplantation, acute fulminant liver failure, or prior LT were excluded from the present study. In total, from January 2015 to January 2018, 307 consecutive patients with cirrhosis who underwent LT at the First Affiliated Hospital, School of Medicine, Zhejiang University were included. Data regarding recipient age, sex, presence of hepatocellular carcinoma (HCC), MELD score, body mass index (BMI), Child-Pugh grading score, pretransplant international normalized ratio (INR), albumin, total bilirubin (TB), plasma creatinine (Pcr), blood urea nitrogen (BUN), and serum CystC were retrospectively collected.
Estimated GFR
The eGFRs were commonly estimated by CKD-EPI-Pcr, MDRD-4, MDRD-6, Hoek, CKD-EPI-CystC, and CKD-EPI-Pcr-CystC equations (6-9). All equations are listed in Table 1. As the Pcr, CyctC, and urea nitrogen were the most frequently encountered parameters for assessing renal function, the CKD-EPI-Pcr, MDRD-6, and CKD-EPI-CystC equations based on those three parameters were selected in the present study for further evaluation.

Full table
Based on the eGFR calculated by the CKD-EPI-CystC equation, patients were divided into four groups according to the Kidney Disease Outcomes Quality Initiative (KDOQI) classification (10): stage I (normal renal function, eGFR ≥90 mL/min/1.73 m2), stage II (60≤ eGFR <90 mL/min/1.73 m2), stage III (30≤ eGFR <60 mL/min/1.73 m2) and stage IV–V (eGFR <30 mL/min/1.73 m2).
Follow-up data
A standardized follow-up protocol was adopted for all patients, in which the end-point was patient death or graft loss. Patients who were lost to follow-up were considered dead. All data were analyzed anonymously and de-identified prior to analysis. The data was updated to October 12th, 2018.
Ethical statement
Written informed consent was obtained from all participants. Ethical approval was obtained from the Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University, and in accordance with the ethical guidelines of the 1975 Declaration of Helsinki.
Statistical analysis
The variables were expressed as median with interquartile range (IQR) or number (percentage). Survival curves were analyzed using the Kaplan-Meier method with the log-rank test. Cox regression was carried out to evaluate the risk factors for patient survival. Variables reaching 10% significance in univariate analysis were included in the multivariate analysis. P<0.05 was considered to be statistically significant. All statistical analyses were performed with statistical software package SPSS19.0 (SPSS Inc, Chicago, IL, USA).
Results
Distribution and clinical characteristics of patients
In total, 307 patients were included in the present study. A total of 265 patients (86.3%) were male, and the median age of the study group was 50 years old (mean 49.7 years old; range, 24–74 years old). A total of 252 recipients (82.1%) were infected with the hepatitis B virus (HBV), and 110 (35.8%) were diagnosed with HCC. The median follow-up time was 15.5 months (mean 17.8 months; range, 0–41.8 months).
The median eGFR according to the CKD-EPI-Pcr, MDRD-6, and CKD-EPI-CystC equations were 103.88 mL/min/1.73 m2 (mean 100.43 mL/min/1.73 m2; range 12.17–153.80 mL/min/1.73 m2), 213.71 mL/min/1.73 m2 (mean 222.22 mL/min/1.73 m2; range 24.19–534.67 mL/min/1.73 m2), and 77.92 mL/min/1.73 m2 (mean 77.59 mL/min/1.73 m2; range 5.66–150.86 mL/min/1.73 m2) respectively. Distribution and clinical characteristics of patients according to KDOQI classification based on CKD-EPI CystC equation are presented in Table 2.
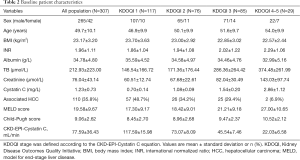
Full table
Patient survival
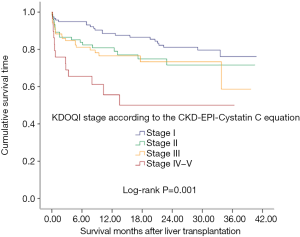
Patients with higher KDOQI stage based on the CKD-EPI-CystC equation were found to have a shorter survival time (Figure 1, P=0.001). The 3-month, 1- and 3-year survival rates in KDOQI stage I based on CKD-EPI-CystC were 94.8%, 88.5%, and 76.1%, respectively; 86.5%, 80.9%, and 71.6% in stage II, respectively; 84.7%, 76.5%. and 58.7% in stage III, respectively; and 69.0%, 55.6%, and 50.0% in stage IV–V, respectively (P=0.001). In univariate analysis for post-transplant mortality, MELD score (P=0.046), eGFR according to MDRD6 equation (P=0.006), CKD-EPI-Pcr equation (P=0.004), and CKD-EPI-CystC equation (P<0.001) were found to be associated with increased risk of mortality (Table 3). Although associated HCC was not found to be significant for mortality in univariate analysis (P=0.502), based on our previous experience, it had significant impact on post-transplant survival and therefore was brought into multivariate analysis. After multivariate analysis, three independent prognostic predictors for patient survival were identified: MELD score [hazard ratio (HR) =1.035; 95% confidence interval (CI), 1.006–1.066; P=0.018], associated HCC (HR =2.314; 95% CI, 1.253–4.273; P=0.007), and KDOQI stage III–V according to CKD-EPI-CystC equation (Table 3).
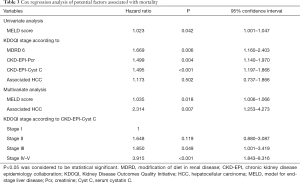
Full table
Conclusions
To the best of our acknowledge, the present study was the first large, single-center study based on a Han population which evaluated the association between preoperative serum CystC with post-transplant mortality. The results confirmed that eGFR prior to transplantation according to CKD-EPI-CystC equation had significant prognostic value for patient survival. Recipients with higher preoperative eGFR were expected to have a higher survival rate. Creatinine-based equations, including MDRD-6 and CKD-EPI-Pcr, were found to have no value for predicting postoperative patient survival.
CystC, known as cysteine protease inhibitor C, was considered an ideal indicator for accurate assessment of renal function. Released into the blood by all nucleated cells at a steady rate, serum CystC is eliminated exclusively by glomerular filtration and then completely reabsorbed and catabolized by the proximal tubular epithelial cells, instead of going back to the blood. In a previous study, the concentration of serum CystC was not influenced by age, sex, muscle volume, serum bilirubin, malignant tumor, drugs, etc. and only affected by renal function (11). The half-life of CystC was shorter than creatinine (1.5 vs. 4–5 hours), which made it more accurate for reflecting rapid changes in renal function (12). Owing to the implementation of international reference material, present commercial CystC assays were highly concordant, which made the measurement of CystC precise and reproducible (13). In patients with advanced liver disease, studies supported the notion that CystC-based equations were more accurate and reliable than creatinine-based ones (5,14).
Primary or secondary renal dysfunction is prevalent in patients before and after LT. It was proposed that renal function evaluated by CystC-based eGFR had a significant correlation with long-term survival of liver recipients (15,16). Thus, in our study, we divided the patients into four groups according to the KDOQI stage based on the eGFR calculated by CKD-EPI-CystC equation. The results showed that patients in KDOQI stage I had a 1-year survival rate of 83%, while for those patients in the KDOQI stage IV–V only 25% survived more than 1 year. These results are consistent with previous reports and suggest that pretransplant renal function may be a significant predictor for patient mortality after transplantation, and that renal function can be best described by eGFR based on CKD-EPI-CystC equation.
The MELD scoring system, introduced in 2002, incorporated plasm creatinine, TB, and the INR, and has been proven to be a simple and objective tool for quantifying the clinical status of potential liver transplant recipients (17). According to the criterion, LT should be considered when the MELD score is above 15 (18,19). In accordance with previous studies, the multivariate analysis of our study confirmed that MELD score was independently associated with decreased survival. However, Kim et al. stated that a few initial variables of the MELD score had not been completely proven to have prognostic values while other effective variables, such as CystC in this study, have not been taken into account for this model (19). With regards to assessing the discrepancy resulting from an excessive proportion of serum creatinine and INR in the MELD score equation (20), it might be plausible to replace creatinine with CystC for calculating MELD score, a measure which is worth further evaluating.
A few limitations of the present study needed to be mentioned. The first is that we did not use a gold standard method to assess the actual value of GFR. Another limitation is that the information for postoperative complications, such as acute or chronic renal dysfunction, infection, and primary liver graft dysfunction, was not recorded or further analyzed. However, the aim of our study was assessing the prognostic value of CystC for liver recipient’s survival, instead of detecting the accuracy of CystC for determining actual GFR. These limitations did not, however, compromise the integrity of the present work.
In conclusion, MELD score and preoperative eGFR based on the CKD-EPI-CystC equation were both independent risk factors for long-term survival after LT. Patients in a higher KDOQI stage determined by pretransplant serum CystC, rather than Pcr, was expected to have a lower survival rate. These findings suggest that CystC might be the better index for replacing creatinine in calculating MELD score.
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China (No. 81572307, 81773096), Major Project of Medical and Health Technology Development Program in Zhejiang Province (No. 7211902), Medical Health Science and Technology Project of Zhejiang Provincial (No. 2016KYB083), Public Welfare Technology Research Project of Zhejiang Province (No. LGD19C040006), General Research Project of the Zhejiang Provincial Education Department (No. Y201840044), and Clinical Medicine Innovation Center of Precision Diagnosis and Treatment for Hepatobiliary and Pancreatic Disease of Zhejiang University (No. 2017-02-06).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. All participants had signed written informed consent. Ethical approval was obtained from the Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University. All data were analyzed anonymously and identified prior to analysis. Written informed consents for publication are obtained from all participants. Detail has been removed from these case descriptions to ensure anonymity. The editors and reviewers have seen the detailed information available and are satisfied that the information backs up the case the authors are making.
References
- Merion RM. Current status and future of liver transplantation. Semin Liver Dis 2010;30:411-21. [Crossref] [PubMed]
- Aranzana EM, Coppini AZ, Ribeiro MA, et al. Model for End-Stage Liver Disease, Model for Liver Transplantation Survival and Donor Risk Index as predictive models of survival after liver transplantation in 1,006 patients. Clinics (Sao Paulo) 2015;70:413-8. [Crossref] [PubMed]
- Weber ML, Ibrahim HN, Lake JR. Renal dysfunction in liver transplant recipients: evaluation of the critical issues. Liver Transpl 2012;18:1290-301. [Crossref] [PubMed]
- Gonwa TA, Jennings L, Mai ML, et al. Estimation of glomerular filtration rates before and after orthotopic liver transplantation: evaluation of current equations. Liver Transpl 2004;10:301-9. [Crossref] [PubMed]
- De Souza V, Hadj-Aissa A, Dolomanova O, et al. Creatinine- versus cystatine C-based equations in assessing the renal function of candidates for liver transplantation with cirrhosis. Hepatology 2014;59:1522-31. [Crossref] [PubMed]
- Hoek FJ, Kemperman FA, Krediet RT. A comparison between cystatin C, plasma creatinine and the Cockcroft and Gault formula for the estimation of glomerular filtration rate. Nephrol Dial Transplant 2003;18:2024-31. [Crossref] [PubMed]
- Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007;53:766-72. [Crossref] [PubMed]
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604-12. [Crossref] [PubMed]
- Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20-9. [Crossref] [PubMed]
- K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39:S1-266. [PubMed]
- Zahran A, El-Husseini A, Shoker A. Can cystatin C replace creatinine to estimate glomerular filtration rate? A literature review. Am J Nephrol 2007;27:197-205. [Crossref] [PubMed]
- Sjostrom P, Tidman M, Jones I. The shorter T1/2 of cystatin C explains the earlier change of its serum level compared to serum creatinine. Clin Nephrol 2004;62:241-2. [Crossref] [PubMed]
- Grubb A, Horio M, Hansson LO, et al. Generation of a new cystatin C-based estimating equation for glomerular filtration rate by use of 7 assays standardized to the international calibrator. Clin Chem 2014;60:974-86. [Crossref] [PubMed]
- Mindikoglu AL, Dowling TC, Weir MR, et al. Performance of chronic kidney disease epidemiology collaboration creatinine-cystatin C equation for estimating kidney function in cirrhosis. Hepatology 2014;59:1532-42. [Crossref] [PubMed]
- Uguen T, Jezequel C, Ropert M, et al. Pretransplant renal function according to CKD-EPI cystatin C equation is a prognostic factor of death after liver transplantation. Liver Int 2016;36:547-54. [Crossref] [PubMed]
- Allen AM, Kim WR, Therneau TM, et al. Chronic kidney disease and associated mortality after liver transplantation--a time-dependent analysis using measured glomerular filtration rate. J Hepatol 2014;61:286-92. [Crossref] [PubMed]
- Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000;31:864-71. [Crossref] [PubMed]
- Kamath PS, Kim WR. The model for end-stage liver disease (MELD). Hepatology 2007;45:797-805. [Crossref] [PubMed]
- Kim HJ, Lee HW. Important predictor of mortality in patients with end-stage liver disease. Clin Mol Hepatol 2013;19:105-15. [Crossref] [PubMed]
- Sharma P, Schaubel DE, Sima CS, et al. Re-weighting the model for end-stage liver disease score components. Gastroenterology 2008;135:1575-81. [Crossref] [PubMed]


