
Newborn screening: Taiwanese experience
Introduction
Newborn screening (NBS) is recognized as a highly effective public health program to detect certain diseases before long term health consequences occur. With advances in screening technologies such as the introduction of tandem mass spectrometric (MS/MS) analysis and available therapeutic options, the number of diseases included in NBS programs has undergone rapid expansion in recent years. In Taiwan, NBS program officially started in 1985 with two screening laboratories including National Taiwan University Hospital (NTUH) Newborn Screening Center. The initial screening included phenylketonuria (PKU) and congenital hypothyroidism (CH), but soon homocystinuria (HCY), galactosemia (GAL), and glucose-6-phosphate dehydrogenase (G6PD) deficiency were added. The screening coverage rose exponentially and exceeded over 98% in 1996. A new screening condition—congenital adrenal hyperplasia (CAH), and a new technique—tandem mass spectrometry (MS/MS) were first added to the NTUH program in 2001 and 2002, respectively, and soon were opened to the whole population. In 2007, the government adapted 11 conditions as the recommended panel, established the cost of testing and provides a subsidy. For those with low socioeconomic status, the government covers the full cost of testing. The government also puts emphasis on the timeliness of newborn-screening, setting benchmarks for the efficient collection, transportation, testing, and reporting the results. The recommended timeliness for the high-risk babies is to report and communicate the results to the newborn’s healthcare provider within 6–8 days of life.
Pompe NBS
NTUH introduced the first program to implement Pompe disease NBS in 2005 (1) to investigate the benefits of early diagnosis and early treatment with recombinant human acid α-glucosidase (GAA) in infantile-onset Pompe disease (IOPD). Blood samples were collected from the 3rd day of life. In this program, GAA, neutral α-glucosidase (NAG), and maltase-glucoamylase (MGA) activities were measured in separate fluorometric assays using commercially available fluorogenic (4-methylumbelliferone) substrates (2). The latter two enzyme activities are used to normalize the protein amount in extracts to help separate true Pompe-positive from false-positive samples (1). In 2008, we first reported NBS for Pompe disease in Taiwan and described the identification of 3 cases of classic IOPD after screening 132,538 newborns (1). Soon after, Pompe NBS was included in the original Taiwan NBS panel. The program also identifies babies with GAA deficiency and biallelic (likely) pathologic mutations but no heart involvement at birth, a group of possible later-onset Pompe disease (LOPD) patients (3). In 2015, we switched to the MS/MS format because of its multiplex capacity (4).
The evolution of NBS methods
Our first report (1) noted a relatively high false positive rate that was later confirmed to be due to the pseudodeficiency allele (p.G576S) in the GAA gene (5). This variant is prevalent in people of Asian descent (6,7) including Japanese, Chinese, and Koreans. Cells from a subject homozygous for this amino acid substitution exhibited 15% and 11% of the normal enzyme activity levels for an artificial substrate and glycogen, respectively (6). This variation may modify the in cis pathologic variation (8) and result in a severe phenotype. The prevalence of this variation in homozygosity is around 3% in Asian populations (7,9,10). Because we sequenced the GAA gene in all cases that screened positive, we were able to confirm that some cases were, indeed, false-positive based on the genotype and biochemical phenotype (GAA enzyme activity) (5). We have developed strict cutoffs to improve the performance of the screen without missing LOPD diagnosis while maintaining the timeliness of IOPD detection. The revised algorithm using a NAG/GAA cutoff ratio ≥60 produced a positive predictive value (PPV) of 63.4%, and adding the percentage of acarbose inhibition as a second-tier test (for those with inconclusive results) further improved the PPV to 92.9% (9). Similar screening algorithms and adjustment to separate pseudodeficiency cases from true Pompe disease have lowered the false-positive rate in Japan (11).
In 2015, we started the MS/MS LSD multiplex assay, including Pompe disease, mucopolysaccharidosis (MPS) I, Gaucher disease, and Fabry disease (4). Two-tier cutoffs remained because the positive prediction value (PPV) by only GAA activity was not satisfied. The PPV using the critical cutoff at 0.5 µM/h would be 71.4% (5 patients out of a total of 7 newborns who have GAA activity <0.5 µM/h), and at 0.7 µM/h be 14.7% (5 patients out of a total of 34 newborns who have GAA activity <0.7 µM/h), respectively. Of these 5 patients, one had IOPD and 4 were suspected to have LOPD. However, IOPD shown in that dataset had 0.44 µM/h GAA DBS activity, and therefore a cutoff at 0.7 µM/h should be safer to overcome the variation of the test and the samples. As for the 192 borderline cases with GAA activity between 0.7–1.2 µM/h, only one individual with GAA activity of 0.75 µM/h was predicted to be a LOPD patient. Without our two-tier cutoffs, the MS/MS LSD multiplex assay would have poor PPV (0.5%) and false positive rate of 0.3%. Programs using only one tier will need to trade between possible false negatives and high false positives. Our 2nd tier strategy increases the PPV to 9%. Alternatively, programs need to repeat sampling (12) or applying a 2nd tier test such as genotyping (13), thus increasing the cost of screening or risking the delay in treatment. Recently, a new 2nd tier test marker—the creatine/creatinine ratio (14)—has been proposed with promising results.
Confirmation and treatment algorithm
The timeliness requirement for Taiwan NBS (established in 2007) has benefited the performance of Pompe NBS program. Our revised algorithm allowed to avoid repeat sampling and shorten the diagnosis age, especially in LOPD. The diagnosis and treatment algorithm are shown in Figure 1. Because the DBS enzyme activity assay cannot predict the phenotype, cardiac involvement needs to be evaluated. This includes chest X-ray, electrocardiogram (EKG), creatine kinase (CK) levels, pro-B-type natriuretic peptide (pro-BNP) levels, and heart echocardiogram (15). Blood samples for genotyping, measuring GAA activity, and determination of cross-reactive immunologic material (CRIM) status are collected. For babies with heart involvement, treatment with recombinant human GAA (rhGAA) is initiated immediately after confirmation of GAA deficiency and positive CRIM status either by genotyping prediction or by western blotting using the blood sample. Enzyme replacement therapy (ERT) is scheduled for the next day after the heart involvement is confirmed, unless immunomodulation therapy to prevent anti-GAA antibodies production is planned. For babies without heart involvement, treatment is delayed until abnormalities appear in the follow-up period.
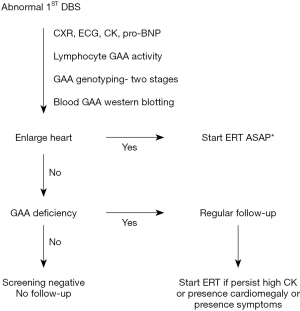
Treatment
The treatment initiation time and the dosage of rhGAA have evolved with time. In 2006, the 1st IOPD patient detected through NBS program had the diagnosis at age 19 days immediately after the 1st abnormal NBS result. ERT with rhGAA was started at age 26 days after the complete workup. The efficiency of NBS samples collection and transportation has improved since 2007; the 3rd IOPD patient was diagnosed at age 9 days and started on ERT at age 17 days. The accumulated knowledge moved us to faster confirmation and ERT application process. Since 2009 the patients could start ERT the day after confirmation as shown in Figure 1. However, due to the residual muscle involvement, persistently elevated CK, plateau in scores of muscle motor function evaluations, ptosis, and speech disorders (see the following section) even in this earliest ERT cohort, the dosage of rhGAA was increased to 40 mg/kg/qow (or equally 20 mg/kg/qw) since 2012. The decision was made at the age 4–6 years in the first 5 patients (ERT starting in 2006 and 2007 at 12–34 days), at the age 2–4 years in the following 5 patients (ERT starting in 2009 to 2011 at 6–16 and 48 days in the prematurity) (16,17), and at 1 year of age in 2 patients (ERT starting in 2012 and after). The serial muscle MRI (18) provided some helpful insights. First, although the biopsies showed no glycogen storage and the CK levels were (nearly) normal after 6 months of ERT (15), high intensity areas over the quadriceps on T2-weighted and short-tau inversion recovery images remained. Second, some of the signal changes appeared to be reversible by higher rhGAA dose commenced by age 2 years. Therefore, since 2016 all newly diagnosed IOPD patients received a double dose of the drug (40 mg/kg/qow). Neither the dosage nor the initiation time was correlated with the titers of anti-drug antibodies.
Survival
In 2009, we first reported the benefit of NBS for IOPD patients. The 5 classical IOPD patients identified by NBS were compared to a group of 10 IOPD patients who were symptomatic at diagnosis and had been treated from the age of 2–6 months (15). The 5 newborns came in for confirmation at age 7–33 days and received their first infusion of rhGAA 1–7 days after diagnosis. NBS group showed a 100% short-term ventilator-free survival and earlier independent walking age. With more classic IOPD patients treated from birth and longer follower-up duration, the benefit of NBS remained as shown in Figure 2. The overall survival and the invasive ventilator-free survival remained as 100% in the NBS group (Figure 2). Compared to the first 5 patients (15) who have been treated at a median age of 26 days (range, 12–34 days), the following 5 patients have been treated even earlier at a median age of 14 days (range, 6–48 days) due to the overall efficacy improvement and the accumulated experience in the confirmation process.
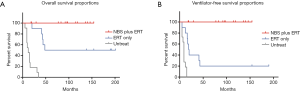
It is necessary to emphasize that the patients identified by NBS in Taiwan till now are confirmed (16) or predicted (19) to be CRIM-positive. The anti-rhGAA antibody titers were low in the patients in our study (median maximal titer value of 1:1,600 over a range from undetectable to 1:12,800 (16), which was similar to the titers in patients diagnosed clinically and treated beyond the newborn period (20). Nevertheless, the regular monitoring of the anti-rhGAA antibody titers is necessary. A case in point is a patient who later developed a sustained intermediate titer as high as 1:25,600 and therefore received immunomodulation therapy to reduce the titer to 1:3,200.
Emerging phenotypes
Muscle weakness over the pelvic girdle gradually appeared in the patients after 2 years of age and was correlated with an increase in CK levels. Ptosis was present in half of the patients, and speech disorders were common (16,17). Increase in the dosage of rhGAA improved partially the speech quality (17), implying the inadequate treatment effect. Another evidence of limited efficacy comes from the muscle MRI studies—all of them show signal changes and later muscle atrophy, indicating the residual myopathy (18). Abnormalities in brain myelination (16,21-23) were a common finding both in newborn- and non-newborn-treated IOPD survivors. Early initiation of ERT was correlated with a high probability of normal independent walking age, less muscle MRI involvement scores (18), and better speech quality (17), but the emerging pattern was still present, even with a high dose of rhGAA (40 mg/kg/qow or 20 mg/kg/week). The limitations of the current therapy highlight the need to test higher dosage earlier, as well as the need for more effective second-generation ERT or gene therapy.
Current situation
Since DBS enzyme activity measurement can detect both IOPD and LOPD patients, the overall incidence of Pompe disease from our NBS data is 1 in 17,000, including an incidence of classic IOPD of 1 in 52,000 and of other types of 1 in 25,000 (9). The second Pompe screening program in Taiwan used fluorescent methods followed by MS/MS analysis, and it identified a similar incidence of Pompe disease (approximately 1 in 15,000) (12). The incidence of IOPD by NBS and by clinical experience is similar, making NBS valuable not only for providing early treatment for IOPD but also for detecting underdiagnosed LOPD or even asymptomatic GAA-deficient individuals.
Those who tested positive but don’t present cardiomyopathy at birth are classified as “suspect” LOPD patients. We recommend monitoring both muscular and cardiac conditions closely such as every 3 months in the first year. Although cardiomyopathy is not a common finding in LOPD, we still need to be careful about the atypical subtype that presents with cardiomyopathy after the newborn stage (24). Starting from 1 year of age, the Peabody Developmental Motor Scale, Second Edition (PDMS-II) was administered every 12 months along with the measurements of CK and urine Glc4. ERT was initiated when a significant persistent elevation of CK was detected or with the onset of considerable motor delay (3).
In our program, we identified 19 LOPD patients by 2011 (9). Six of the 19 total cases (32%) were placed on rhGAA treatment at ages 1.5–36 months (3,25). Four of the 6 have known genotypes and elevated CK. The other 2 patients have novel genotypes (3), normal CK, and normal baseline urinary Glc4 (26). However, all muscle biopsies before ERT initiation showed abnormalities associated with glycogen storage including autophagic defects and lipofuscin inclusions (25,27). After 6-month of therapy, most of those changes were absent, suggesting that these patients are the greatest beneficiaries of early recognition. Currently they are at age of 8–13 years, with normal motor milestones development, normal physical activity, and normal respiratory function, although three of them still have persistent elevation of CK. As for the remaining 13 patients, the long-term follow-up revealed normal CK, normal urine Glc4, and normal physical activities. These cases are likely to represent a mild phenotype or variants with the conflicting interpretations of pathogenicity.
Variants of unknown significance (VUS)
The most common VUS detected in our cohort is c.[752C>T; 761C>T] (p.[S251L; S254L]), or dual mutation (3). This dual mutation is a rare variant located at the conserve region, resulting in a very low GAA activity in vitro using the artificial substrate or glycogen (20), and it is classified as likely pathogenic by the latest ACMG criteria. The dual mutation has been observed alone or in combination with c.1411_1414del (p.E471Pfs*5) in our experience. Another VUS, c.1958C>A (p.T653N), also showed a significantly reduced enzyme activity in vitro and was classified as likely pathogenic. However, we recently encountered a 40-year-old female presenting with mild proximal muscle weakness who has these c.[752C>T; 761C>T] and c.1958C>A in trans. Although GAA deficiency was demonstrated in the lymphocytes and in fibroblasts, she had normal CK, normal urine Glc4 levels, and normal muscle morphology on quadriceps biopsy. We also encountered a 40-year-old male who harbors the dual mutation in combination with another known pathologic IOPD mutation in trans; this asymptomatic individual has completely normal muscle strength, normal CK and urine Glc4, and normal muscle MRI. Therefore, the babies with this dual mutation need a long follow-up before the judgment can be made about the potential benign character of this dual mutation.
Conclusions
NBS for Pompe disease can be performed by a variety of methods, but the main method relies on enzyme activity measurement. As a result, both classic severe IOPD and less-severe LOPD patients are identified by screening. Early detection and ERT are beneficial to classic severe IOPD patients, but current therapy has limitations, in particular with respect to the skeletal and neurologic manifestations of the disease. A combination of early detection, close monitoring and early ERT is likely to be beneficial to less-severe LOPD patients, but adequate genetic counseling, education and support for both parents and patients should be encouraged.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chien YH, Chiang SC, Zhang XK, et al. Early detection of Pompe disease by newborn screening is feasible: results from the Taiwan screening program. Pediatrics 2008;122:e39-45. [Crossref] [PubMed]
- Chamoles NA, Niizawa G, Blanco M, et al. Glycogen storage disease type II: enzymatic screening in dried blood spots on filter paper. Clin Chim Acta 2004;347:97-102. [Crossref] [PubMed]
- Chien YH, Lee NC, Huang HJ, et al. Later-onset Pompe disease: early detection and early treatment initiation enabled by newborn screening. J Pediatr 2011;158:1023-7.e1. [Crossref] [PubMed]
- Chiang SC, Chen PW, Hwu WL, et al. Performance of the Four-Plex Tandem Mass Spectrometry Lysosomal Storage Disease Newborn Screening Test: The Necessity of Adding a 2nd Tier Test for Pompe Disease. Int J Neonatal Screen 2018;4:41. [Crossref]
- Labrousse P, Chien YH, Pomponio RJ, et al. Genetic heterozygosity and pseudodeficiency in the Pompe disease newborn screening pilot program. Mol Genet Metab 2010;99:379-83. [Crossref] [PubMed]
- Tajima Y, Matsuzawa F, Aikawa S, et al. Structural and biochemical studies on Pompe disease and a "pseudodeficiency of acid alpha-glucosidase". J Hum Genet 2007;52:898-906. [Crossref] [PubMed]
- Kroos MA, Mullaart RA, Van Vliet L, et al. p.[G576S; E689K]: pathogenic combination or polymorphism in Pompe disease? Eur J Hum Genet 2008;16:875-9. [Crossref] [PubMed]
- Yang CC, Chien YH, Lee NC, et al. Rapid progressive course of later-onset Pompe disease in Chinese patients. Mol Genet Metab 2011;104:284-8. [Crossref] [PubMed]
- Chiang SC, Hwu WL, Lee NC, et al. Algorithm for Pompe disease newborn screening: results from the Taiwan screening program. Mol Genet Metab 2012;106:281-6. [Crossref] [PubMed]
- Shigeto S, Katafuchi T, Okada Y, et al. Improved assay for differential diagnosis between Pompe disease and acid alpha-glucosidase pseudodeficiency on dried blood spots. Mol Genet Metab 2011;103:12-7. [Crossref] [PubMed]
- Oda E, Tanaka T, Migita O, et al. Newborn screening for Pompe disease in Japan. Mol Genet Metab 2011;104:560-5. [Crossref] [PubMed]
- Yang CF, Liu HC, Hsu TR, et al. A large-scale nationwide newborn screening program for Pompe disease in Taiwan: towards effective diagnosis and treatment. Am J Med Genet A 2014;164A:54-61. [Crossref] [PubMed]
- Wasserstein MP, Caggana M, Bailey SM, et al. The New York pilot newborn screening program for lysosomal storage diseases: Report of the First 65,000 Infants. Genet Med 2019;21:631-40. [Crossref] [PubMed]
- Tortorelli S, Eckerman JS, Orsini JJ, et al. Moonlighting newborn screening markers: the incidental discovery of a second-tier test for Pompe disease. Genet Med 2018;20:840-6. [Crossref] [PubMed]
- Chien YH, Lee NC, Thurberg BL, et al. Pompe disease in infants: improving the prognosis by newborn screening and early treatment. Pediatrics 2009;124:e1116-25. [Crossref] [PubMed]
- Chien YH, Lee NC, Chen CA, et al. Long-term prognosis of patients with infantile-onset Pompe disease diagnosed by newborn screening and treated since birth. J Pediatr 2015;166:985-91.e1-2.
- Zeng YT, Hwu WL, Torng PC, et al. Longitudinal follow-up to evaluate speech disorders in early-treated patients with infantile-onset Pompe disease. Eur J Paediatr Neurol 2017;21:485-93. [Crossref] [PubMed]
- Peng SS, Hwu WL, Lee NC, et al. Slow, progressive myopathy in neonatally treated patients with infantile-onset Pompe disease: a muscle magnetic resonance imaging study. Orphanet J Rare Dis 2016;11:63. [Crossref] [PubMed]
- Yang CF, Yang CC, Liao HC, et al. Very Early Treatment for Infantile-Onset Pompe Disease Contributes to Better Outcomes. J Pediatr 2016;169:174-80.e1. [Crossref] [PubMed]
- Chien YH, Hwu WL, Lee NC. Pompe disease: early diagnosis and early treatment make a difference. Pediatr Neonatol 2013;54:219-27. [Crossref] [PubMed]
- Chien YH, Lee NC, Peng SF, et al. Brain development in infantile-onset Pompe disease treated by enzyme replacement therapy. Pediatr Res 2006;60:349-52. [Crossref] [PubMed]
- Ebbink BJ, Aarsen FK, van Gelder CM, et al. Cognitive outcome of patients with classic infantile Pompe disease receiving enzyme therapy. Neurology 2012;78:1512-8. [Crossref] [PubMed]
- Ebbink BJ, Poelman E, Plug I, et al. Cognitive decline in classic infantile Pompe disease: An underacknowledged challenge. Neurology 2016;86:1260-1. [Crossref] [PubMed]
- Lee DH, Qiu WJ, Lee J, et al. Hypertrophic cardiomyopathy in pompe disease is not limited to the classic infantile-onset phenotype. JIMD Rep 2014;17:71-5. [Crossref] [PubMed]
- Chien YH, Lee NC, Huang PH, et al. Early pathologic changes and responses to treatment in patients with later-onset Pompe disease. Pediatr Neurol 2012;46:168-71. [PubMed]
- Chien YH, Goldstein JL, Hwu WL, et al. Baseline Urinary Glucose Tetrasaccharide Concentrations in Patients with Infantile- and Late-Onset Pompe Disease Identified by Newborn Screening. JIMD Rep 2015;19:67-73. [Crossref] [PubMed]
- Feeney EJ, Austin S, Chien YH, et al. The value of muscle biopsies in Pompe disease: identifying lipofuscin inclusions in juvenile- and adult-onset patients. Acta Neuropathol Commun 2014;2:2. [Crossref] [PubMed]


