
C1Q/TNF-related protein 4 expression correlates with herpes simplex encephalitis progression
Introduction
Herpes simplex encephalitis (HSE) is associated with high mortality, having about a 70% mortality in untreated patients, and a 30% mortality even in the early stages of adequate-antiviral-agent-treated patients (1-3). The infection mainly undermines the temporal and frontal lobes or limbic system. Pathological progress includes edema, hemorrhage, neuronal degeneration, and necrosis in the brain parenchyma (4). Additionally, HSE survivors are often accompanied by severe and permanent neurological damage and focal neurological deficits, such as various degrees of cognitive, memory, and behavioral deficits (5).
Herpes simplex virus is classified as herpes simplex virus 1 and herpes simplex virus 2. Most HSE cases are caused by herpes simplex virus 1 (90%) (6). The primary infection of HSV-1 is often located in the oropharynx, especially gingivostomatitis (7). HSV-1 can cause lip herpes, herpetic keratitis, herpetic dermatitis, and encephalitis. HSE occurs at any age. There are 1 to 3 million people with HSE and it accounts for 5–10% of the viral encephalitis cases in the USA (8).
The mechanisms of HSE pathogenesis has not been fully understood. Studies have shown both viral infection and brain damage is caused by the progression of HSE (9). The innate immune response to the virus is a double-edged sword. On the one hand, the immune response activates the anti-inflammatory system which protects the nervous system (10). On the other hand, nerve damage induced by the excessive immune response is the key reason for disability and death (11,12). There are many pro-inflammatory factors involved in the progression of HSE such as IL-6 and TNF-α. In a mice model, C1Q/TNF-related protein 4 (CTRP4) concentration in peripheral blood was highly consistent with the concentration in brain tissue homogenate levels (13). CTRP4 plays an important role in innate immune response (14), and CTRP4 has been demonstrated to be anti-inflammatory in the colitis model. Evidence has shown that CTRP4 is closely related to inflammatory cytokine through effecting the STAT3 and NF-kB signal pathways (15). However, the function of CTRP4 in HSE is still unclear.
In the present study, we hypothesized that CTRP4 plays an important role in HSE progression. CTRP4 was found highly expressed in HSE by bioinformatics analysis. Using ELISA, we then found that the concentration of CTRP4, IL-6, and TNF-α was highly detected in the HSE serum when compared to others; IHC stained CTRP4 in normal brain, HSE, tuberculous meningitis (TBM), and bacterial meningitis (BM) brain tissues. Mini-mental state examination (MMSE) clinical scores and MRI imaging was evaluated using the different stages of the HSE patients. Data showed a similar tendency to the previous studies. These findings suggest that CTRP4 might act as a potential disease severity index for HSE.
Methods
Ethics statement
This study was approved by the Ethics Committee of Beijing Tiantan Hospital, Beijing Ditan Hospital, and Capital Medical University (NO. KYSQ 2019-011-01). Informed consent was obtained from all patients, and the study was conducted following the ethical standards of the Helsinki Declaration.
Patients sample collection
Serum was obtained from 30 normal donors, 15 HSE patients, 5 BM patients, and 6 TBM patients. One case of HSE had cerebral hemorrhage and cerebral palsy. The patient received a craniotomy and necrotic brain tissue was removed to study the pathology.
There was 1 case of bacterial encephalitis, 1 case of TBM and 1 sample of normal brain tissue which came the from donors. After individual informed consents were obtained from patients and normal persons, venous blood was collected in sterile tubes. 100 µL of blood sample was used for determining CTRP4, IL-6, and TNF-α with ELISA.
Bioinformatics analysis
The gene expression data of GSE51040 were downloaded. We analyzed 23 mice data from hippocampi infected with HSE and 23 normal mice. The gene expression data of GSE6509 were downloaded. We analyzed 20 cases of LPS-induced bacterial encephalitis mice and 9 normal mice. These gene expression data of GSE23074/GSE29507 were also downloaded. We analyzed 10 patients with tuberculous encephalitis and 4 normal persons.
Enzyme-linked immunosorbent assay (ELISA)
Blood samples were collected and naturally coagulated at room temperature for 20 minutes. After centrifugation at 1,000 ×g for 20 minutes at 4 °C, the supernatant was carefully collected and stored at −80 °C. CTRP4 (MM-1640H2), IL-6 (MM-0049H1), and TNF-α (MM-0122H1) ELISA kits were purchased from Meimian Biotechnology (Yancheng, Jiangsu, China). Serum samples (100 µL) were analyzed three times.
The individual reference and patient samples were added to the microtiter plate. The standard concentration was determined as: 4,000, 2,000, 1,000, 500, 250, and 125 pg/mL. One hundred µL of serum was added to the appropriate wells according to the manufacturer instructions. One hundred µL of the enzyme conjugate was added to the appropriate wells and incubated for 1h at RT. Each plate was washed 4 times, and 50 µL of each substrate (liquid A and B) was added to each well. Next, the substrates were incubated for 15 minutes at 37 °C. Fifty µL of the stopping solution was added to each well, and the plate was tapped gently to mix thoroughly. The optical density (O.D.) was read at 450 nm using a microplate reader (Bio-Rad, Hercules, CA, USA).
Immunohistochemistry (IHC) staining
Human brain tissue paraffin sections were obtained as described previously. We stained 4-µm-thick sections with IHC. For IHC staining, primary antibody-CTRP4 from Proteintech (Rabbit Polyclonal, 1:100, Catalog number: 14023-1-AP, USA) was used, and the Polymer HRP Detection System was bought from Bioss (Rabbit, PV-0023). The DAB Immunohistochemistry Color Development Kit was purchased from BBI (E670033-0100). Each presented immunohistochemistry was repeated at least three times. Images were acquired with a microscope (magnify, ×400).
Clinical data collection
Related clinical information was collected, including gender, age, clinical symptoms, CTRP4 concentration, cerebrospinal fluid (CSF) protein, CSF leucocytes, and MRI. HSE patients were graded with MMSE clinical scores on admission. MMSE can comprehensively, accurately, and quickly reflect the mental state of the subjects and the degree of their cognitive impairment. MMSE was a general guideline for the progression of HSE progress. Patients enrolled had a similar educational background. Then, we analyzed the correlation between this data and the CTRP4 expression.
Statistical analysis
Data were presented as the mean ± SD and analyzed with SPSS 17.0. One-way analysis of variance or the repeated measures were used to judge the statistical significance of the differences, and a Spearman’s rank correlation coefficient for bivariate correlations between CTRP4 and inflammatory markers was completed. Values of *P<0.05, **P<0.01, and ***P<0.001 were considered statistically significant.
Results
Bioinformatics analysis
CTRP4 showed a higher expression in HSE than in normal brain tissues in the mice (Figure 1). The expression of CTRP4 in HSV-1 infected brain (mean intensity =27,754.7) was higher than that of control samples (mean intensity =9,473.0), and the fold change was 2.93 (P=0.019). CTRP4 showed a comparable level of expression between BM (mean intensity =783.26) and normal brain (mean intensity =876.71) tissue in mice (P=0.7143). There was no significant difference between BM and the normal group. Similarly, CTRP4 showed a comparable level of expression between TBM (mean intensity =2,662) and normal brain (mean intensity =6,339) tissue. In human tissues (P=0.0946), there was no significant difference between the TBM and normal group.
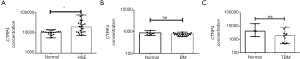
ELISA
Expression of CTRP4 was higher in HSE serum than in the normal serum (P<0.001), the BM serum, and the TBM serum. The average CTRP4 value in the normal serum was 775.804, 1,559.387 in the HSE serum, 826.2 in the TBM serum, and 772.5 in the BM serum. In the HSE serum, the average IL-6 value was 10.3265, and the average TNF-α value was 20.386. The correlation coefficient of CTRP4 and IL-6 was 0.937, and the correlation coefficient of CTRP4 and TNF-α was 0.945 (Figure 2).

Validation data in the patients’ cohort
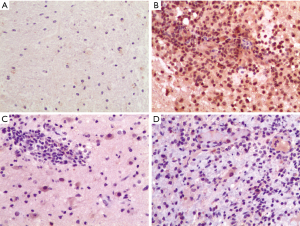
Expression of CTRP4 was higher in the HSE brain sample than the normal brain sample, BM brain sample, and TBM brain sample in humans. CTRP4 showed strong positive staining in HSE brain, but weak staining in the normal brain, TBM brain, and BM brain (Figure 3).
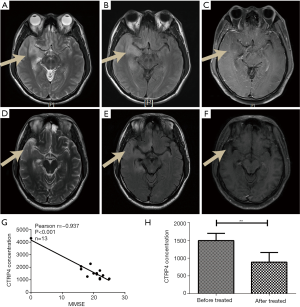
Correlation of CTRP4 expression with progression of HSE
The patient MRI showed long T1, and T2 signal accompanied high CTRP4 and serious syndrome before treatment. An MRI of the same patient showed shrinking lesions that were accompanied by lower CTRP4 with syndromes being relieved after treatment.
We analyzed the detailed clinical information including the MMSE table from the 14 HSE patients (Table 1), and significant correlations were found between CTRP4 concentration and MMSE scores (Figure 4).
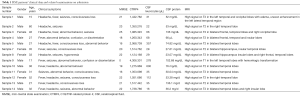
Full table
Discussion
HSE is a life-threatening condition with high mortality as well as a significant morbidity rate in survivors. Histopathologically, HSV causes hemorrhagic necrosis, accompanied by neuronal intranuclear inclusion bodies (INIBs) (4,16). Although the pathogenesis of HSE remains unclear, viral-infection-induced excessive inflammation is believed to play a crucial role. IL-6 and TNF-α are the most common pro-inflammatory cytokines (17,18). The IL-6/STAT3 axis plays an important role in the pathogenesis of HSE. The study showed that IL-6 combined with the STAT3 signaling cascades played an important role in the inflammatory reaction. During the early stage of an HSV-1 infection, STAT3 had protective effects by mediating cellular responses to multiple cytokines, governing gene expression and regulating the development and activation of immune cells (19).
In HSE animal models, studies showed that HSE inhibitors could specifically suppress HSV-induced activation of inflammation factors (20). IL-6 protects from HSE likely through its downstream STAT3 cascade (17). In mice models, STAT3 took part in HSE progression. STAT3 KO mice were more susceptible to an HSV-1 infection compared to the wild type mice. STAT3 pathway modulates HSV-1 reactivation and governs its infections (18,21-23). Phosphorylated STAT3 was suggested to be correlative with protective effects in the analysis of many transcription factors.
CTRP family is suggested to be involved in inflammation (16,20). The structure of CTRP4 includes two globular domains, whereas the other CTRPs contain only one globular domain. CTRP4 is produced as a classical secreted protein. Research showed that the overexpression of CTRP4 could increase IL-6 expression and activation of STAT3 in HepG2 cells, indicating that CTRP4 and IL-6 are correlatively upregulated during inflammation (15). In dextran sulfate sodium (DSS)-induced acute colitis model, the expression of endogenous CTRP4 was greatly up-regulated, and phosphorylation of STAT3 was downregulated in rhCTRP4-treated mice. Expression of IL-6 and TNF-α and phosphorylation of STAT3 were decreased in the rhCTRP4-treated group when compared to the control group. CTRP4 can trigger the pathway of both NF-kB and IL6/ STAT3 (13,15,24). In coronary heart disease, the serum CTRP4 concentration is elevated correlating with inflammatory factors such as TNF-α and IL-6. CTRP4 was elevated in HSE serum compared to TBM, BM, and normal serum. In HSE, CTRP4 concentration showed similar kinetics with other inflammatory factors such as IL-6 and TNF-α. The high expression of CTRP4 correlated with the patient's symptoms, MRI imaging, and MMSE clinical score. It might be that CTRP4 takes part in the regulation of innate immune response in the human brain.
Elevated levels of neutrophil lymphocyte ratio (NLR) were found to be associated with a poor survival rate of patients who had undergone a coronary artery bypass graft (25). Many cancer survival studies have suggested that NLR is a significant predictor of overall and disease-specific survival of patients (26,27). Systemic inflammation measured by NLR has a significant association with prevalent chronic conditions (28). We found a correlation between NLR and CTRP4 level (Figure 2F). The neutrophil-lymphocyte ratio could be an important measure of systemic inflammation as it is cost effective, readily available, and can be calculated easily. The potential value of CTRP4 in predicting the outcome of HSE needs further investigation.
Conclusions
In summary, CTRP4 is closely correlated with HSE progression and may serve as an indicator of the disease’s severity. The mechanism of CTRP4 in HSE may be related to IL-6-mediated inflammatory responses including STAT3 signaling. Moreover, the mechanism of CTRP4 remains to be further studied. Nevertheless, our study highlights the potential significance of CTRP4 in the progression of HSE and provides a novel intervention perspective in the treatment and prognosis of HSE.
Acknowledgments
Funding: This study was supported by the Beijing Key Laboratory Project (No. TMCD201603).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of Beijing Tiantan Hospital, Beijing Ditan Hospital, and Capital Medical University (NO. KYSQ 2019-011-01) and written informed consent was obtained from all patients.
References
- Granerod J, Ambrose HE, Davies NW, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis 2010;10:835-44. [Crossref] [PubMed]
- Lo WB, Wilcock DJ, Carey M. Neurological picture. Herpes encephalitis complicated by cerebral haemorrhage. J Neurol Neurosurg Psychiatry 2013;84:1404-6. [Crossref] [PubMed]
- Whitley RJ. Herpes simplex virus infections of the central nervous system. Encephalitis and neonatal herpes. Drugs 1991;42:406-27. [Crossref] [PubMed]
- Liu H, Qiu K, He Q, et al. Mechanisms of Blood-Brain Barrier Disruption in Herpes Simplex Encephalitis. J Neuroimmune Pharmacol 2019;14:157-72. [Crossref] [PubMed]
- Steiner I. Herpes simplex virus encephalitis: new infection or reactivation? Curr Opin Neurol 2011;24:268-74. [Crossref] [PubMed]
- Hjalmarsson A, Blomqvist P, Skoldenberg B. Herpes simplex encephalitis in Sweden, 1990-2001: incidence, morbidity, and mortality. Clin Infect Dis 2007;45:875-80. [Crossref] [PubMed]
- Kolokotronis A, Doumas S. Herpes simplex virus infection, with particular reference to the progression and complications of primary herpetic gingivostomatitis. Clin Microbiol Infect 2006;12:202-11. [Crossref] [PubMed]
- Kohl S. Herpes simplex virus encephalitis in children. Pediatr Clin North Am 1988;35:465-83. [Crossref] [PubMed]
- Lafaille FG, Pessach IM, Zhang SY, et al. Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature 2012;491:769-73. [Crossref] [PubMed]
- Yissachar N, Zhou Y, Ung L, et al. An Intestinal Organ Culture System Uncovers a Role for the Nervous System in Microbe-Immune Crosstalk. Cell 2017;168:1135-48.e12. [Crossref] [PubMed]
- Colten HR. Immunology. Drawing a double-edged sword. Nature 1994;371:474-5. [Crossref] [PubMed]
- Fimia GM, Stoykova A, Romagnoli A, et al. Ambra1 regulates autophagy and development of the nervous system. Nature 2007;447:1121-5. [Crossref] [PubMed]
- Byerly MS, Petersen PS, Ramamurthy S, et al. C1q/TNF-related protein 4 (CTRP4) is a unique secreted protein with two tandem C1q domains that functions in the hypothalamus to modulate food intake and body weight. J Biol Chem 2014;289:4055-69. [Crossref] [PubMed]
- Wang L. CTRP4: a new member of the adipocytokine family. Cell Mol Immunol 2017;14:868-70. [Crossref] [PubMed]
- Li Q, Wang L, Tan W, et al. Identification of C1qTNF-related protein 4 as a potential cytokine that stimulates the STAT3 and NF-kappaB pathways and promotes cell survival in human cancer cells. Cancer Lett 2011;308:203-14. [Crossref] [PubMed]
- Baumann RJ, Walsh JW, Gilmore RL, et al. Brain biopsy in cases of neonatal herpes simplex encephalitis. Neurosurgery 1985;16:619-24. [Crossref] [PubMed]
- Chucair-Elliott AJ, Conrady C, Zheng M, et al. Microglia-induced IL-6 protects against neuronal loss following HSV-1 infection of neural progenitor cells. Glia 2014;62:1418-34. [Crossref] [PubMed]
- Hsia HC, Stopford CM, Zhang Z, et al. Signal transducer and activator of transcription 3 (STAT3) regulates host defense and protects mice against herpes simplex virus-1 (HSV-1) infection. J Leukoc Biol 2017;101:1053-64. [Crossref] [PubMed]
- Zhang H, Hu H, Greeley N, et al. STAT3 restrains RANK- and TLR4-mediated signalling by suppressing expression of the E2 ubiquitin-conjugating enzyme Ubc13. Nat Commun 2014;5:5798. [Crossref] [PubMed]
- Chen D, Su A, Fu Y, et al. Harmine blocks herpes simplex virus infection through downregulating cellular NF-kappaB and MAPK pathways induced by oxidative stress. Antiviral Res 2015;123:27-38. [Crossref] [PubMed]
- Chucair-Elliott AJ, Gurung HR, Carr MM, et al. Colony Stimulating Factor-1 Receptor Expressing Cells Infiltrating the Cornea Control Corneal Nerve Degeneration in Response to HSV-1 Infection. Invest Ophthalmol Vis Sci 2017;58:4670-82. [Crossref] [PubMed]
- Du T, Zhou G, Roizman B. Modulation of reactivation of latent herpes simplex virus 1 in ganglionic organ cultures by p300/CBP and STAT3. Proc Natl Acad Sci U S A 2013;110:E2621-8. [Crossref] [PubMed]
- Yu CR, Dambuza IM, Lee YJ, et al. STAT3 regulates proliferation and survival of CD8+ T cells: enhances effector responses to HSV-1 infection, and inhibits IL-10+ regulatory CD8+ T cells in autoimmune uveitis. Mediators Inflamm 2013;2013:359674. [Crossref] [PubMed]
- Fan H, Zhang M, Li S, et al. WITHDRAWN: C1qTNF related protein 4 (CTRP4) promotes pancreatic ductal adenocarcinoma cell proliferation and migration via activation of EGFR-PI3K-NF-kappaB pathway. Oncol Res 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Gibson PH, Croal BL, Cuthbertson BH, et al. Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. Am Heart J 2007;154:995-1002. [Crossref] [PubMed]
- Walsh SR, Cook EJ, Goulder F, et al. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 2005;91:181-4. [Crossref] [PubMed]
- Sarraf KM, Belcher E, Raevsky E, et al. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg 2009;137:425-8. [Crossref] [PubMed]
- Imtiaz F, Shafique K, Mirza SS, et al. Neutrophil-lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in the Asian population. Int Arch Med 2012;5:2. [Crossref] [PubMed]


