
Quantitative gait analysis for laser cue in Parkinson’s disease patients with freezing of gait
Introduction
Gait performance are severely impaired in Parkinson’s disease (PD) patient with freezing of gait (FOG) (PD + FOG). The striking features of gait impairments in PD are characterized by bradykinesia (1), akinesia (1) and freezing (2). Svehlík et al. investigated gait performance in twenty PD patients and found abnormal gait in spatiotemporal parameter, kinematics, and kinetics (3). Patients walked with a shortened step length and prolonged double stance time in spatiotemporal parameter, a significantly reduced range of motion (ROM) in kinematics in the ankle, knee and hip joints, a reduced push-off power generation in ankle and lift-off power generation in hip in kinetics (3). These gait deficiencies may lead to a high rate of falling with the complications, i.e., fracture, confining to bed, infection, and then seriously reduce the quality of life. However, drugs including Botulinum toxin type B (4) show less effect on freezing in PD + FOG.
For PD + FOG, visual cues have been suggested to improve gait abnormality through bypassing the impaired basal ganglia, thus relieving emphasis on automatic motor function (5,6). Parallel stripes lines on the ground has been advocated as the most effective way to improve gait deficiency (7,8). The benefits of parallel stripes visual cue cueing on kinematics and kinetics have been well documented. Lewis et al. showed that the gait spatiotemporal parameters, the kinematic and kinetic changes increased to controls with visual cueing (5). Morris et al. showed that external visual cueing might contribute to ameliorate the spatiotemporal and kinematic deficiencies (9,10) across lower limbs while impaired kinetics also existed, i.e., reduced push-off power generation at ankle joint (9). Sidaway et al. demonstrated that visual cues might increase ROM in hip and knee joints and engender more stable motion control in the lower limb (8). Lee et al. found that visual cues significantly increased the extents of pelvic tilt, knee flexion, and ankle dorsiflexion (11).
Although visual cueing which were provided by parallel stripes glued on the pathway could significantly ameliorate gait performance in PD + FOG, unfortunately, it could not apply in daily life. At present, laser visual cue is the most conveniently available modality of visual cue and has been gradually utilized to improve gait performance (i.e., stride length, walking velocity, etc.) and overcome freezing in PD + FOG (12-16). Buated et al. showed that laser cue (LC) from lase line with a cane significantly increased both stride length and walking velocity (12). An open-label study of 26 PD patients with FOG by Donovan et al. (13) found that the LC showed the modest efficacy in reducing freezing and falls. In a recent study of seven PD + FOG demonstrated that the Mobilaser attached to a 4-wheeled walker has a potential effect in alleviating stride length and freezing (14).
However, the negative effects of LC have also been found. Bunting-Perry et al. found that laser visual cue in patients with PD was not able to improve step length, velocity or freezing episodes (15). Kompoliti et al. compared gait performance (i.e., step velocity and freezing episodes) among different conditions (i.e., unassisted walking, inverted stick and laser beam stick) and indicated that LC did not show any significant benefits than other two conditions in PD + FOG (16).
To our knowledge, although studies of laser cueing on gait in PD + FOG were further explored (5,17), few studies have revealed the effects of laser cueing on gait kinematics-kinetics, especially in PD + FOG. Whether laser visual cueing may alleviate gait kinematics-kinetics remains unclear. Therefore, the purpose of this study is to describe and examine the changes of gait performance in PD + FOG under the LC condition, thus making up for the lack in this field. The patients in no laser cue (NC) condition (baseline) and healthy aged persons were used as controls. Such a method may present deeper insight into the effects of LC on gait performance.
Methods
Subjects
Forty patients were enrolled and thirty-four patients (19 males, 15 females) completed the study. Six patients dropped out of the test due to hip fracture (1 patient), apoplexy (3 patients) and pre-exposure to LC prior to test (2 patients).
All patients were tested during the “OFF” state with their own preferable shoes. To achieve a relative “OFF” state, the patient was instructed to stop taking short-acting dopaminergic drug no less than 12 hours and long-acting medications at least 24 hours beforehand (18). Inclusion criteria were as follows: PD + FOG according to the United Kingdom Parkinson’s Disease Society Brain Bank (UKPDSBB) clinical diagnostic criteria (19); H&Y (Hoehn-Yahr) stages II–IV; the ability of walking 10-meter at least 6 times without any auxiliary orthosis. The patients were excluded if they had other central neurological system disease; severe medical disease affecting gait (i.e., cardiothoracic disorders, mental or visual disorders); and the Mini-Mental State Examination (MMSE) score less than 23 (20).
Thirty-two health controls (HC) included 17 men and 15 women without neurologic, motor and other medical sickness affecting gait. The age, weight, and height in HC were comparable to PD + FOG (Table 1).
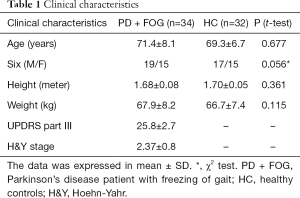
Full table
All subjects were required to sign written informed consent prior to the test. The study was approved by the Ethics Committee of the Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine.
Experimental process
Gait trials were performed utilizing an eight-camera optical motion capture system (Vicon Nexus 1.8.5, VICON, Oxford Metrics Ltd., UK) with a sampling frequency of 100 HZ and three AMTI force platforms (Advanced Mechanical Technology Inc., Watertown, MA, USA) with a sampling frequency of 1,000 HZ mounted midway on the walkway (10 m × 0.7 m).
Infrared-reflexive markers were located on the anatomical bony landmarks and shoes corresponding to anatomical bony landmarks on feet. These points included the left/right calcaneus apophyseal, the left/right fifth metatarsal head, the left/right point between the first and the second metatarsal head, left/right first metatarsal head, left/right lateral/medial malleolus, left/right lateral/medial supracondylar of femur, left/right greater trochanter of femur, left/right posterior superior iliac spines (PSIS) and left/right anterior superior iliac spines (ASIS). The trace cluster markers were located on the middle of the left/right thigh/shank respectively and the position two centimeters above and below the bilateral PSIS. Virtual 3D software was applied to manually divide gait cycle events and to analyze gait performance (i.e., spatiotemporal, kinematic and kinetic data). Joint internal moments (Nm) and powers (watts) were normalized according to the subject’s body mass (kilograms). In this study, we compared the more-affected leg side in patients with the left leg in HC subjects. The more-affected leg side was determined by questionnaire. In this study, the more-affected leg side were the right leg in 14 patients and the left leg in 20 patients.
The LC was provided by laser line projected from a laser generator attached over the sternum on the ground one step length prior to the patient’s feet. All patients were tested at their usual self-selected speed in NC cue first and then were tested under LC condition. In LC condition, patients were instructed to step on the laser line. Subjects repeated six times in each condition from which the average spatiotemporal parameter, sagittal plane kinematic and kinetic data were obtained.
All subjects were asked to take a rest for about ten minutes in case of fatigue during the test.
Statistical analysis
Outcome measures obtained from this study were described as sagittal plane kinematic and kinetic parameters of the hip, knee, and ankle joints, as well as spatiotemporal parameters (walking velocity, cadence, stride length, single and double stance time). We used one-way analysis of variance (ANOVA) to compared difference in spatiotemporal and kinematic parameters among NC, LC, and HC. Bonferroni test for post-hoc analysis was performed. Due to walking velocity regarded as a covariant factor, Covariance analysis was applied to compare the kinetic parameters among NC, LC, and HC. All statistical analyses were performed by SPSS 19.0. Significance level was set at 0.05.
Results
Spatiotemporal parameters
Table 2 showed the spatiotemporal data in PD + FOG and health controls. PD + FOG in NC condition showed significantly decreased stride length and velocity whereas cadence was higher than HC (stride length: NC vs. HC =0.93±0.20 vs. 1.1±0.12; velocity: NC vs. HC =0.87±0.17 vs. 1.12±0.13; cadence: NC vs. HC =131±14.75 vs. 116±9.37, P=0.001 respectively). Double stance phase of gait cycle was also prolonged in PD + FOG with NC condition than HC (NC vs. HC =27.83±3.62 vs. 24.36±2.19, P=0.001). While in LC condition, the values of these parameters were significantly improved in comparison with NC (stride length: LC vs. NC =1.06±0.18 vs. 0.93±0.20, P=0.027; velocity: LC vs. NC =1.01±0.19 vs. 0.87±0.17, P=0.045; cadence: LC vs. NC =120±13.26 vs. 131±14.75, P=0.035; double support time: LC vs. NC =25.16±3.18 vs. 27.83±3.62, P=0.041) and were comparable to HC.
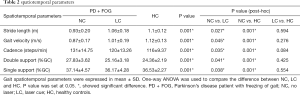
Full table
Kinematic parameters
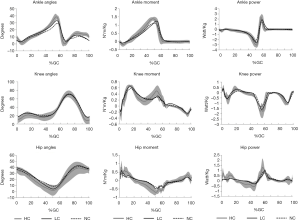
The ROM in the lower-limb joints (ankle, knee and hip) were all reduced in PD + FOG in NC (Table 3, Figure 1). The difference of kinematics parameters was most pronounced in the ankle and hip joints between NC and LC conditions.
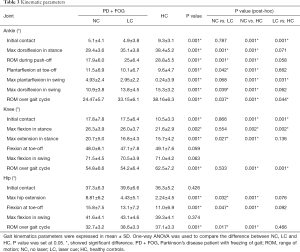
Full table
The amplitude of ankle maximum dorsiflexion in the stance and swing phase were remarkably lower in NC than HC (P=0.001, 0.001). ROM in ankle during push-off was also significantly reduced in NC compared with HC (P=0.001). Consequently, a marked reduction of plantarflexion in ankle at toe-off were observed in NC (P=0.001). While the value of maximum dorsiflexion in stance and swing phase and the ROM in ankle during push-off were significantly improved in LC condition in PD + FOG compared with NC condition (P=0.001, 0.039, 0.001, respectively). Although the amplitude of plantarflexion in swing phase was also increased in LC condition, no significant difference was found between LC and NC conditions (P=0.068). Additionally, the dorsiflexion amplitude at initial contact in LC condition was comparable to that in NC condition (P=0.787).
The amplitude of maximum knee extension in stance phase was significantly increased in LC condition in comparison with NC condition (P=0.027). While the ROM of knee during gait cycle was comparable between NC and LC conditions (P=0.533).
The extent of maximum hip extension was considerably higher in LC condition than that in NC condition (P=0.032) and was comparable to HC (P=0.076). The ROM of hip during gait cycle in LC condition was also comparable to that in HC (P=0.466).
Kinetic parameters
More kinetic changes in lower limb joints was observed (Table 4, Figure 1). In ankle joint, the moment at loading response in both NC and LC conditions were significantly decreased compared with HC (P=0.001, 0.001), while the loading response moment in ankle was comparable between NC and LC conditions (P=0.672). However, the maximum extensor moment during stance were significantly increased in LC condition than in NC condition (P=0.028), and were comparable compared with HC (P=0.067). Power generation in the pre-swing phase was significantly improved in LC condition than in NC condition (P=0.039), however, it was still significantly lower than HC (P=0.037).
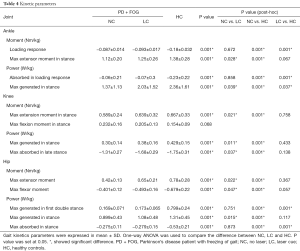
Full table
In knee joint, both the maximum extensor moment and the power generation during stance were significantly larger in LC condition than in NC condition (P=0.021, 0.011), and also no significant difference in them were found between LC condition and HC (P=0.758, 0.433). However, the flexor moment during the single stance phase showed no significant difference among LC, NC and HC (P=0.068). The maximum power absorption in late stance phase was significantly higher in LC condition than in NC condition (P=0.037).
In the hip joint, the maximum hip extensor moment in LC condition was significantly higher than in NC condition (P=0.022) as well as the maximum hip flexor moment (P=0.047). The power generation in the first double stance was comparable between LC and NC conditions (P=0.751). While the power generation in pre-swing phase was significantly improved in LC condition compared with NC condition (P=0.015). The power absorption in pre-swing phase showed no significant difference between LC and NC conditions (P=0.873).
Discussion
This study showed the quantitative analysis of LC on the gait performance (spatiotemporal, kinematic and kinetic variables) in PD + FOG. Although there were still some gait abnormalities in LC condition compared with HC, LC was beneficial to improve the gait performance for PD + FOG.
Spatiotemporal parameters
The key spatiotemporal findings in this study showed that LC could significantly improve spatiotemporal parameters. Consistent with previous studies, the spatiotemporal features of gait in PD + FOG in NC condition were characterized by slower velocity, shorter stride length and higher cadence (3,21).While in LC condition, PD + FOG showed a significantly slower cadence, longer stride length and faster velocity than that in NC condition, which was also consistent with previous study (12,22). Apparently, PD + FOG may walk with a comparable gait spatiotemporal pattern to HC when LC is provided. Increased stride length and slower cadence are beneficial to PD + FOG since they may contribute to prevent the occurrence of “sequence effect” (sequence effect: as the patient advances, the stride length or stride time becomes smaller and smaller), thereby contributing to reduce FOG (23).
The shortened double support time was also found in PD + FOG in LC condition compared with NC condition. Increased double support time may be a sign of instability (3). Postural instability can have a marked impact on the gait pattern in PD + FOG. The maintenance of dynamic stability in PD + FOG is achieved by postural synergies. Prolonging the time of double support during gait cycle means increasing time to achieve gait stability, thereby minimizing the needs for postural control system (9). Additionally, patients overcome the fear of falling by increasing double support time, thereby regain gait stability (9). Therefore, it was suggested that LC might increase gait stability in PD + FOG.
Kinematics
In agreement with previous studies (3,10,21), the ROM value of the joints in lower limbs was remarkably reduced in NC condition compared with HC. In contrast, LC considerably enlarged the joint angular excursions, especially in ankle and hip joints. Gait deficiencies in PD + FOG arise from a mismatch between cortically selected movement amplitude and the actual size of lower limb movements executed during walking (10). In LC condition, LC might draw the attention to walking task and invoke a more conscious motor control strategy that bypasses the impaired basal ganglia (5), therefore making the lower limbs flexion-extension easier. Additionally, approaching stripes might have certain dynamic properties that encourage stepping over or on the stripes, thus improve the ankle and hip joints angular excursions. Further, RL cue provided a chance for vision on the lower limbs to compensate for a proprioception processing deficit, therefore planed ankle and hip movement at a cortical level (22).
In LC condition, the increased ROM during push-off resulted from both the maximum dorsiflexion in stance phase and plantarflexion at toe-off at ankle joint. The increase of lower limbs joints movement might be the basis of the stride length lengthening. The factors, such as the improvement of ROM at ankle joint during push-off, maximum hip and knee extension in stance phase, likely contributed to prolong the stride length.
Kinetics
Gait kinematic improvement is caused by the amelioration of kinetics. Striking kinetic improvements in the lower-limb joints were also found in LC. The ankle dorsiflexion moment at loading response were significantly lower in NC condition compared with HC, which was also consistent with previous studies (3,5). The “flat-foot” gait in PD + FOG may be related to a reduce heel rocker, inadequate knee extension or limited hip flexion. However, in this study, ankle moment at loading response did not show significant improvement in LC condition. A possible explanation was that, since the PD + FOG subjects were observed to step on the laser line with their toes instead of contacting ground with heel, therefore, the vector of ground reaction force still remained close to the ankle joint. Thus, the walking pattern make the ankle loading respond moment comparable between LC and NC conditions.
In this study, LC could significantly improve the ankle maximum plantarflexion moment in stance phase and power generation in pre-swing phase. Increased ROM in ankle plantarflexion during push-off also results in improvement of power generation during push-off. Judge et al. indicated that the power generation in plantar-flexor had the strongest effect on predicting the stride length in elderly subjects (24). Feebleness or diminished activation of plantar-flexor muscles, increased muscle stiffness might lead to decreased power generation at the ankle (25). In fact, PD patients do not lose the ability to produce healthy gait patterns, but have difficulty in activating motor control system. Laser line can draw attention on the walking pattern, thus unloading the emphasis on automatic motor function through alternative visual-motor circuits (5). Additionally, approaching the laser line has certain dynamic properties that encourage stepping over or on the line (26).
A significant increased power generation in the knee joint during the single stance phase of the gait cycle was observed in LC condition compared with NC condition, and was even comparable between LC condition and HC. This occurred with a higher knee extension during the stance phase of gait. It seemed that LC might contribute to make subjects generate adequate power to extend and stabilize the knee joint. Additionally, PD + FOG also showed a higher power absorption in the late stance phase of the gait cycle in LC condition. It was suggested that the power absorption in knee was the natural result of energy transferred from plantarflexors in ankle to the knee (24). The increased power absorption at the later stance phase in knee joint in LC condition might be related to the higher energy that is transferred from power generation in ankle during push-off (3). This meant that an increased ankle power generation during push-off in LC condition could cause a higher power absorption in knee joint at the late stance phase.
The maximum flexion moment and power generation in hip joint at the second double stance phase decreased in NC condition and increased in LC condition instead. The results might be due to a larger hip extension amplitude. Additionally, it was proposed that LC might enhance power generation in hip joint during preswing phase to lift lower-limb into swing phase. This manner also might compensate for inadequate power generation in ankle joint during push-off. Sofuwa et al. found that dopaminergic medication did not have the same effect of reinforce power generation in hip joint, thereby compensating for the deficit power generation through ankle joint during push-off (27).
A confusing phenomenon in LC condition is that the hip maximum extensor moment was significantly increased compared with NC condition in the first double stance phase, while the hip power generation in LC condition was comparable to NC condition. Generally, the two kinetic parameters either decrease or increase simultaneously. A possible reason explained for this result was that, since the power generation was the product of the joint moment times angular velocity, the hip angular velocity might influence the power generation.
Implication for clinic
Clinically, PD + FOG tend to show a gait pattern with shortened stride length, slower velocity and decreased ROM in lower limb joints. These gait deficiencies gradually worsen as the disease progress, severely affecting the quality of life. Svehlík indicated that there was positive correlation between the ankle push-off and hip lift-off power generation and gait velocity (3). Morris et al. also demonstrated that the increased power generation of ankle plantarflexor and hip extensor at push-off is the most likely factor for lengthening stride length (9). It is suggested that the strength and compliance of ankle plantarflexor and hip extensor can translate into quantitative improvements in gait pattern so that kinetics in ankle and hip joints during the stance phase may be considerably improved. Our study found that gait feature in LC condition were shown as an increased stride length/gait velocity and an improvement kinematics/kinetics in multi-joint in lower limb. Therefore, it would imply that LC can be used as a physiotherapy method to benefit PD + FOG to improve gait. Ng et al. indicated that stronger ankle plantarflexors might have propel body forward during walking (28). It is suggested that LC might improve muscle strength in ankle and hip joints, even decrease reduce muscle stiffness, thereby enlarging the ROM in lower limb joints and lengthening the step length. For PD + FOG, LC might be an economical and effective way of rehabilitation training in daily life. However, the myoelectrical activity in lower limbs was not considered. Next study should also explore the effects of LC on electromyogram (EMG) and the relationship between myoelectrical activity and muscle generation power.
Sidaway et al. utilized visual cue provided by 1-m strips of 2.5-cm-wide blue masking tape placed on ground to train PD patients for 1 months, and found that gait speed and step length were increased with visual cues, and the improvements in gait speed and step length were still evident 1 month following the removal of the cues (8). Donovan et al. assessed the efficacy of laserlight visual cues for overcoming FOG (13). In the authors’ study, subjects underwent a 1–2-month baseline period of use of a cane or walker without visual cues, followed by 1 month using the same device with the laserlight visual cue. The authors demonstrated that although the magnitude of improvement appears less at week 4, the change at week 4 is not significantly different from the change at weeks 1, 2, or 3. Also, the results do not provide clear evidence for a loss of benefit over time. Whether the kinematic-kinetic improvement of LC may fade over time is still unclear. Longer-term and larger studies may be necessary to identify this possibility.
Conclusions
The results of this study demonstrated that LC could ameliorate the abnormal gait pattern in PD + FOG. In addition to spatiotemporal parameters, kinematics in ankle and hip joints were also improved as well as kinetics. LC may arouse the ability of PD + FOG to take advantage of visual feedback to adjust motion amplitude and then reduce dependence on kinesthetic feedback. Therefore, LC may be promising when applied as an optional technique in the rehabilitation training in PD patients with FOG.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethics Committee of the Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (No. 2017-33). All subjects were required to sign written informed consent prior to the test.
References
- Morris ME, Iansek R, Matyas TA, et al. The pathogenesis of gait hypokinesia in Parkinson's disease. Brain 1994;117:1169-81. [Crossref] [PubMed]
- Fahn S. The freezing phenomenon in parkinsonism. Adv Neurol 1995;67:53-63. [PubMed]
- Svehlík M, Zwick EB, Steinwender G, et al. Gait analysis in patients with Parkinson's disease off dopaminergic therapy. Arch Phys Med Rehabil 2009;90:1880-6. [Crossref] [PubMed]
- Fernandez HH, Lannon MC, Trieschmann ME, et al. Botulinum toxin type B for gait freezing in Parkinson's disease. Med Sci Monit 2004;10:CR282-4. [PubMed]
- Lewis GN, Byblow WD, Walt SE. Stride length regulation in Parkinson's disease: the use of extrinsic, visual cues. Brain 2000;123:2077-90. [Crossref] [PubMed]
- Nonnekes J, Snijders AH, Nutt JG, et al. Freezing of gait: a practical approach to management. Lancet Neurol 2015;14:768-78. [Crossref] [PubMed]
- Vitorio R, Lirani-Silva E, Pieruccini-Faria F, et al. Visual cues and gait improvement in Parkinson's disease: which piece of information is really important? Neuroscience 2014;277:273-80. [Crossref] [PubMed]
- Sidaway B, Anderson J, Danielson G, et al. Effects of long-term gait training using visual cues in an individual with Parkinson disease. Phys Ther 2006;86:186-94. [PubMed]
- Morris ME, McGinley J, Huxham F, et al. Constraints on the kinetic, kinematic and spatiotemporal parameters of gait in Parkinson’s disease. Hum Mov Sci 1999;18:461-83. [Crossref]
- Morris M, Iansek R, McGinley J, et al. Three-dimensional gait biomechanics in Parkinson's disease: evidence for a centrally mediated amplitude regulation disorder. Mov Disord 2005;20:40-50. [Crossref] [PubMed]
- Lee SJ, Yoo JY, Ryu JS, et al. The effects of visual and auditory cues on freezing of gait in patients with Parkinson disease. Am J Phys Med Rehabil 2012;91:2-11. [Crossref] [PubMed]
- Buated W, Sriyudthsak M, Sribunruangrit N, et al. A low-cost intervention for improving gait in Parknson’s disease patients: A cane providing visual cues. Eur Geriatr Med 2012;3:126-30. [Crossref]
- Donovan S, Lim C, Diaz N, et al. Laserlight cues for gait freezing in Parkinson's disease: an open-label study. Parkinsonism Relat Disord 2011;17:240-5. [Crossref] [PubMed]
- Van Gerpen JA, Rucker CT, Matthews M, et al. Lifting the "FOG" with laser generated visual-cueing. Neurologist 2012;18:298-301. [Crossref] [PubMed]
- Bunting-Perry L, Spindler M, Robinson KM, et al. Laser light visual cueing for freezing of gait in Parkinson disease: A pilot study with male participants. J Rehabil Res Dev 2013;50:223-30. [Crossref] [PubMed]
- Kompoliti K, Goetz CG, Leurgans S, et al. "On" freezing in Parkinson's disease: resistance to visual cue walking devices. Mov Disord 2000;15:309-12. [Crossref] [PubMed]
- Egerton CJ, McCandless P, Evans B, et al. Laserlight visual cueing device for freezing of gait in Parkinson's disease: a case study of the biomechanics involved. Physiother Theory Pract 2015;31:518-26. [Crossref] [PubMed]
- Rowe JB, Hughes L, Ghosh BC, et al. Parkinson's disease and dopaminergic therapy--differential effects on movement, reward and cognition. Brain 2008;131:2094-105. [Crossref] [PubMed]
- Hughes AJ, Ben-Shlomo Y, Daniel SE, et al. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology 1992;42:1142-6. [Crossref] [PubMed]
- Dick JP, Guiloff RJ, Stewart A, et al. Mini-mental state examination in neurological patients. J Neurol Neurosurg Psychiatry 1984;47:496-9. [Crossref] [PubMed]
- Albani G, Cimolin V, Fasano A, et al. "Masters and servants" in parkinsonian gait: a three-dimensional analysis of biomechanical changes sensitive to disease progression. Funct Neurol 2014;29:99-105. [PubMed]
- Lebold CA, Almeida QJ. An evaluation of mechanisms underlying the influence of step cues on gait in Parkinson's disease. J Clin Neurosci 2011;18:798-802. [Crossref] [PubMed]
- Chee R, Murphy A, Danoudis M, et al. Gait freezing in Parkinson's disease and the stride length sequence effect interaction. Brain 2009;132:2151-60. [Crossref] [PubMed]
- Judge JO, Davis RB 3rd, Ounpuu S. Step length reductions in advanced age: the role of ankle and hip kinetics. J Gerontol A Biol Sci Med Sci 1996;51:M303-12. [Crossref] [PubMed]
- Kerrigan DC, Todd MK, Della Croce U, et al. Biomechanical gait alterations independent of speed in the healthy elderly: evidence for specific limiting impairments. Arch Phys Med Rehabil 1998;79:317-22. [Crossref] [PubMed]
- Azulay JP, Mesure S, Amblard B, et al. Visual control of locomotion in Parkinson's disease. Brain 1999;122:111-20. [Crossref] [PubMed]
- Sofuwa O, Nieuwboer A, Desloovere K, et al. Quantitative gait analysis in Parkinson's disease: comparison with a healthy control group. Arch Phys Med Rehabil 2005;86:1007-13. [Crossref] [PubMed]
- Ng SS, Hui-Chan CW. Contribution of ankle dorsiflexor strength to walking endurance in people with spastic hemiplegia after stroke. Arch Phys Med Rehabil 2012;93:1046-51. [Crossref] [PubMed]


