
VCAM-1+ macrophages usher hematopoietic stem and progenitor cell to vascular niche “hotspots”
In mammals, evidence suggests that hematopoietic stem and progenitor cells (HSPCs) arise in the aorta-gonad-mesonephros (AGM) region (1), migrate to the liver (2) and then to the bone marrow (BM) where definitive hematopoiesis is established shortly after birth and remains sustained during the rest of the mammal’s life. Previous studies have linked HSPC homing to the fetal liver and adult hematopoietic tissues to β1 integrins (3). In these studies, β1 integrin-deficient HSPCs demonstrated deficiencies in seeding hematopoietic tissues in the fetus and in adults (3). Also, ablating β1 integrin expression resulted in failure of HSPCs to engraft irradiated mice (3). In the absence of this molecule, HSPCs demonstrated reduced adherence to endothelial cells (3). β1 integrin subunits form heterodimers by uniting with α integrin subunits, of these heterodimer units α4 (ITGA4) β1 (ITGB1) (VLA-4) is the one that is most linked to hematopoietic HSPC attachment to stromal-derived extracellular matrix proteins (4). Such interaction is linked to HSPC functions like HSPC homing and lodging (5,6) and occurs between VLA-4 on HSPC and vascular cell adhesion molecule 1 (VCAM-1) on stromal cells (7). In mice, α4 (ITGA4) deletion in HSPC has been reported to be associated with impaired BM homing and short term engraftment (8).
Over the last 20 to 30 years, zebrafish have been extensively used to study hematopoiesis in vertebrate animals (9). Such a model allows large-scale genetic and chemical screening. Similar to vertebrate animals, hematopoiesis in zebrafish occurs in different anatomic sites (9). HSPCs arise from the AGM region in the dorsal aorta 26 h post-fertilization (hpf). At 48 hpf, HSPCs travel through the circulation and seed the caudal hematopoietic tissue (CHT) and then kidney marrow. Once HSPCs reside in these niches, CHT or kidney marrow, their differentiation pattern is determined. For example, in CHT HSPCs differentiate to produce erythroid, myeloid and thromboid cells, whereas HSPCs become lymphoid cells in the kidney marrow in addition to the other lineages produced in CHT. Of note, CHT and kidney marrow in zebrafish are equivalent to murine fetal liver and BM, respectively (9). Within the BM, HSPCs are organized within spatial structures called the stem cell “niche” in which the HSPCs are housed and maintained by self-renewal through symmetrical division (10). In general, there are two major BM niches, the endosteal BM niche and the vascular BM niche. While dormant HSPCs mostly reside in the endosteal BM niche, self-renewing HSPCs are the majority in the vascular niche (10). This vascular niche, which is made of specialized endothelial cells called the BM sinusoidal endothelial cells (BMECs), is linked to pivotal HSPC functions, including HSPC mobilization, homing, and differentiation (11).
Although it is well accepted that HSPCs arise in the AGM region and travel to CHT and kidney marrow in zebrafish, the molecular mechanisms underlying this homing process remain largely unknown. In this paper by Li et al. (12), the authors describe the involvement of VCAM-1+ macrophages in the homing process of HSPCs to the CHT’s vascular niche in zebrafish by taking full advantages of its well-defined hematopoietic differentiation patterns (cell lineages and physical compartment distribution) and genetics (13). Li et al. provide compelling evidence that VCAM-1+ macrophages regulate HSPC homing to the vascular niche in an ITGA4 dependent manner, and they demonstrate these macrophages determine HSPC retention using dynamic and quantitative measurements, which collectively elucidate the molecular mechanism underlying an interplay between macrophage and HSPC during a complex niche homing process (12).
To study HSPC homing determinants in zebrafish, the authors first used the CRISPR-Cas9 system to perform a large-scale genetic screening for mutants with homing defects (extended data Figure 1 of the original manuscript) and mapped the mutations to the ITGA4 locus (extended data Figure 2A,B,C,D,E,F,G,H of the original manuscript). Zebrafish with mutations to ITGA4 demonstrated defects in definitive hematopoiesis in the presence of normal primitive hematopoiesis and vascular development. The number of HSPCs in iTGA4 mutated zebrafish CHT was significantly reduced. Using whole-mount in situ hybridization (WISH) analysis, they found that ITGA4 expression in the CHT was Runx1- (an important transcriptional factor that controls HSPC emergence) and Myb- (a transcription factor that controls HSPC migration in the circulation) dependent. To allow real-time characterization of homing to the CHT region and HSPC retention in CHT, they molecularly engineered a photo-inducible construct that fluoresce green in the Dendra2+ endothelial cells in the AGM while they emitted red fluorescence after the transition of endothelial cells to hematopoietic cells occurred. Red Dendra2+ carrying Runx1 transcripts were found in the CHT region by 48–50 hpf. (extended data Figure 3 of the original manuscript). After successfully confirming the model was working by a markedly reduced numbers of HSPCs in the CHT region of the Runx1 and Myb mutants (extended data Figure 3D of the original manuscript), the authors started to track the route of nascent HSPC migration by a high-resolution imaging system after photo-activation.
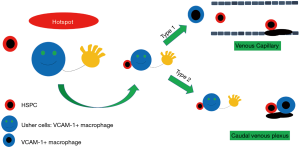
First, the authors examined HSPC retention in the CHT by live-imaging (Figure 1of the original manuscript) by which they demonstrated a clear 3D vascular architecture (Figure 1B & extended data Figure 6 of the original manuscript) and precisely quantified HSPC entry into- and retention within CHT, a critical experimental platform for all subsequent experiments. They discovered that HSPC retention in the CHT was very short in ITGA4 mutants (4 vs. 30 min in wild-type embryos). The authors further identified “hotspots”, areas of an increased frequency of HSPCs within the CHT after monitoring HSPC frequency in the entire CHT over several hours (extended data Figure 4 of the original manuscript) and observed that, once HSPCs arrived hotspots, they decelerated (extended data Figure 5 of the original manuscript). These hotspots were not present in ITGA4 mutants, an observation that was not linked to the vascular system development. Given that VCAM-1 is the major ligand for VLA-4, the authors generated a VCAM-1 mutant. Re-capitulation of HSPC homing and definitive hematopoiesis defects of ITGA4 mutants in VCAM-1 mutants suggested that the ITGA4-VCAM-1 axis controlled the homing and retention of nascent HSPCs.
Interestingly, subsequent immunofluorescent studies showed strong VCAM-1 expression in CHT hotspots, where non-endothelial VCAM-1+ cells were identified next to HSPCs. These cells were identified as VCAM-1+-like macrophages in a macrophage-specific Tg (mpeg1:eGFP) transgenic line and they were found to express macrophage-specific markers by immunofluorescence staining. Depleting macrophages impaired HSPC lodging and definitive hematopoiesis, while restoring VCAM-1 in macrophages almost completely salvaged HSPC retention. The authors finally characterized VCAM-1+ macrophages in the CHT (Figure 3 of the original manuscript) showing evidence that VCAM-1+ macrophages moved along the inner sides of the dorsal caudal venous plexus (CVP) which allowed them to interact frequently and for approximately 30 min with HSPCs as they enter into CHT. The retention (>30 min) in CHT subsequently occurred in 40% of HSPCs, while 60% left CHT. The authors identified three different retention patterns as shown in Figure 4 (original manuscript). In type 1, VCAM-1+ macrophages guided HSPCs to enter the venous capillaries where they stayed longer than 120 min. In type 2, HSPCs interacted with VCAM-1+ macrophages in CVP and then became surrounded by a pocket of endothelial cells. In type 0, HSPCs interacted with VCAM-1+ macrophages longer than 30 min, whereas such interaction did not lead to HSPC migration to the vascular niche. Given their role in guiding HSPCs into the vascular niche, these VCAM-1+ macrophages were called “usher cells” by the authors. These retention patterns reflect the various types of HSPC and VCAM-1+ macrophage interaction surrounding CHT hotspots (Figure 1). In their model (Figure 4A of the original manuscript), it is not clear how the retention types are determined. Alternatively, we speculate that it might be determined by the contact point within the niche where VCAM-1+ macrophages and HSPC interact and by the binding affinity between VLA-4 and VCAM-1. As such, in type 1 the binding strength and affinity between endothelium VCAM-1 and VLA-4 would be stronger than that to macrophage VCAM-1, leading to later release of HSPCs from macrophages and subsequent extravasation into the vascular niche.
Findings from this paper are very important for HSPC research. Firstly, although quantification and imaging of HSPCs in BM was previously shown (14), the well-designed photo-conversion strategies employed by this paper enable a precise measurement of homing and retention of nascent HSPCs within CHT in real-time. Secondly, though it was known that HSPCs were retained in spleen by VCAM-1 (15) and in BM by macrophages (16), this paper provides a solid link between VCAM-1 and macrophage in HSPC retention by depletion and restoration of VCAM-1. Thirdly, the authors overcame the difficulty of dissecting and investigating the contribution of specific cell types to hematopoietic maintenance (17). Fourthly, this study combined molecular approaches with high-resolution microscopic analyses in real-time, which excluded the heterogeneity concern, a major issue of studying the interaction of HSPC with other cell types (17). Fifthly, this paper might have clinical impact in that enrichment of BM VCAM-1+ macrophages before HSPC transplantation might potentially promote initial HSPC retention and result in early homing and engraftment during clinical HSPC transplantation.
This paper combines multidisciplinary strategies to dissect a complex biological mechanism. However, several questions remain unanswered in macrophage-mediated HSPC homing and retention in the vascular niche. For example, are VCAM-1+ macrophages also involved in escorting HSPC in other niches in addition to vesicular niche? Is there any relative difference in VCAM expression between these three types of macrophage-mediated HSPC retention? Are there any additional signals required for type 1 HSPC retention in the vesicular niche? How much does HSPC homing and hotspot retention in the CHT in zebrafish resemble HSPC trafficking to vascular/endosteal niches in mammals? Also, it is of importance to investigate whether HSPC-niche interaction is coordinated with HSPC division, whether dynamic changes of VAL-4/VCAM-1 occur on the endothelium of niches when VCAM-1+ macrophages usher HSPCs to niche, and whether the other chaperones are required for HSPC egress into niches after HSPC releasing from VCAM-1+ macrophages. Results from this study should lead to a better understanding of the cross-talk between HSPCs and VCAM-1+ macrophages surrounding the niches at both cellular and molecular levels.
In conclusion, this study specifically identified VCAM-1+ macrophages as the usher cells to escort HSPCs to the vascular niche using advanced imaging techniques and genetic data. The authors carefully address the role of VCAM-1+ macrophages in guiding photo-activated nascent converted HSPCs within CHT. Their findings enable us to think outside the box of considering macrophages solely as phagocytes. In addition, this study provides direct and compelling evidence to resolve a long-missing puzzle regarding VCAM-1, macrophages and HSPC mobility (5,15,18,19). These findings have implications for advances in stem cell-based medicine, and development of novel therapies for hematological disorders caused by interrupted HSPC and niche interactions (20,21).
Acknowledgments
We thank Dr. Jane Liesveld for her critical review of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 1996;86:897-906. [Crossref] [PubMed]
- Ema H, Nakauchi H. Expansion of hematopoietic stem cells in the developing liver of a mouse embryo. Blood 2000;95:2284-8. [PubMed]
- Potocnik AJ, Brakebusch C, Fässler R. Fetal and adult hematopoietic stem cells require beta1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity 2000;12:653-63. [Crossref] [PubMed]
- Williams DA, Rios M, Stephens C, et al. Fibronectin and VLA-4 in haematopoietic stem cell-microenvironment interactions. Nature 1991;352:438-41. [Crossref] [PubMed]
- Papayannopoulou T, Craddock C, Nakamoto B, et al. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci U S A 1995;92:9647-51. [Crossref] [PubMed]
- Papayannopoulou T, Craddock C. Homing and trafficking of hemopoietic progenitor cells. Acta Haematol 1997;97:97-104. [Crossref] [PubMed]
- Oostendorp RA, Dörmer P. VLA-4-mediated interactions between normal human hematopoietic progenitors and stromal cells. Leuk Lymphoma 1997;24:423-35. [Crossref] [PubMed]
- Scott LM, Priestley GV, Papayannopoulou T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol 2003;23:9349-60. [Crossref] [PubMed]
- Jing L, Zon LI. Zebrafish as a model for normal and malignant hematopoiesis. Dis Model Mech 2011;4:433-8. [Crossref] [PubMed]
- Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol 2006;6:93-106. [Crossref] [PubMed]
- Kopp HG, Avecilla ST, Hooper AT, et al. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 2005;20:349-56. [Crossref] [PubMed]
- Li D, Xue W, Li M, et al. VCAM-1+ macrophages guide the homing of HSPCs to a vascular niche. Nature 2018;564:119-24. [Crossref] [PubMed]
- Wattrus SJ, Zon LI. Stem cell safe harbor: the hematopoietic stem cell niche in zebrafish. Blood Adv 2018;2:3063-9. [PubMed]
- Nombela-Arrieta C, Pivarnik G, Winkel B, et al. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol 2013;15:533-43. [Crossref] [PubMed]
- Dutta P, Hoyer FF, Grigoryeva LS, et al. Macrophages retain hematopoietic stem cells in the spleen via VCAM-1. J Exp Med 2015;212:497-512. [Crossref] [PubMed]
- Winkler IG, Sims NA, Pettit AR, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 2010;116:4815-28. [Crossref] [PubMed]
- Boyerinas B, Sipkins DA. HSPCs in the balance: The vascular niche. Cell Stem Cell 2010;7:645-6. [Crossref] [PubMed]
- Chow A, Lucas D, Hidalgo A, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med 2011;208:261-71. [Crossref] [PubMed]
- Papayannopoulou T, Priestley GV, Nakamoto B. Anti-VLA4/VCAM-1-induced mobilization requires cooperative signaling through the kit/mkit ligand pathway. Blood 1998;91:2231-9. [PubMed]
- Wu L, Mo W, Zhang Y, et al. Impairment of hematopoietic stem cell niches in patients with aplastic anemia. Int J Hematol 2015;102:645-53. [Crossref] [PubMed]
- Medinger M, Krenger W, Jakab A, et al. Numerical impairment of nestin(+) bone marrow niches in acute GvHD after allogeneic hematopoietic stem cell transplantation for AML. Bone Marrow Transplant 2015;50:1453-8. [Crossref] [PubMed]


