Surgical intervention in renal cell carcinoma patients with lung and bronchus metastasis is associated with longer survival time: a population-based analysis
Introduction
Owing to the rising incidence and mortality, malignant neoplasms have become a global health problem. It is estimated that the number of new diagnosed kidney cancer cases and cancer-caused death cases will be 403,262 (2.2%) and 175,098 (1.8%) in 2018, respectively (1). Among those primary kidney neoplasms, renal cell carcinoma (RCC) represents 80–85% cases, which maintains an increasing trend in recent years (2). In this population, a number of patients (5–45%) are suffering from lung and bronchus metastasis (LBM) (3,4). As the most common metastasis site in metastatic RCC (mRCC) patients, LBM represents a late stage and a poor prognosis. In recent decades, the increasing frequency of LBM caused by the development of improved diagnostic techniques, increased survival rate, and life expectancy of cancer patients has gained increased traction.
To date, the most applicable management of RCC patients with LBM is a longstanding debate. In National Comprehensive Cancer Network (NCCN) clinical practice guidelines of kidney cancer (version 4.2018, April 23, 2018), RCC with LBM is classified as stage IV in which nephrectomy and resection of lung metastases are first-line therapy. Cytoreductive nephrectomy (CN) is recommended for patients with multiple metastatic sites. For patients with surgically unresectable condition, molecular targeted therapy may bring survival benefits. In most of previous literature, the small patient population and the lack of existing studies lead to a limited understanding of clinical strategies. Therefore, exploring the impact of clinical intervention on the prognosis of LBM patients in RCC can provide a theoretical basis for the establishment of reliable prognostic indices of these patients.
Surveillance, Epidemiology and End Results (SEER) database is a high-quality database with a rigorous quality assurance program, and thus it is suitable to tackle the problems of limited patients sample size. We conducted the present study to determine the impact of clinical intervention on prognosis of RCC patients with LBM by means of analysis the data from the SEER database.
Methods
We downloaded urinary cancer data [1973–2015] from the SEER database, which covers approximately 26% of the USA population (seer.cancer.org; download date: 2018-08-20) (5). The malignant neoplasms of the kidney was classified was according to the International Classification of Diseases 10 recode C64.9 and one of four clinically relevant RCC-specific histologic subtype statuses: (I) clear cell (8310/3: clear-cell adenocarcinoma, not otherwise specified (NOS); 8322/3: water clear-cell adenocarcinoma; 8313/3: clear-cell adenocarcinoma); (II) papillary (8260/3: papillary adenocarcinoma, NOS); (III) chromophobe (8317/3: RCC, chromophobe type; 8270/3: chromophobe carcinoma); or (IV) collecting duct (8319/3: collecting duct carcinoma). We included patients with a diagnosis of RCC as the first diagnosis using histology codes in order to exclude patients diagnosed with urothelial carcinoma (supplementary Appendix, http://jurology.com/). For each patient, demographics characteristics, for instance, age, gender, race, region and cancer characteristics including histology, causes of death to site record, tumor grade, surgery, and overall survival (OS) time were extracted from SEER database.
Patients diagnosed with carcinoma in situ and benign tumor were excluded. Patients diagnosed at autopsy or through death certificate and who had an unknown LBM or follow-up status were also removed.
Statistical analysis
The primary objective was to compare OS for the RCC patients with LBM treated with surgery versus without surgery. Baseline characteristics were compared using the χ2 test for the categorical variables. The survival analysis was estimated using the Kaplan-Meier (K-M) method and univariate comparison were performed using the log-rank test and unadjusted Cox proportional hazards regression models. Multivariate Cox proportional hazards regression survival models were adjusted for factors including age, gender, race, region, grade of differentiation, and surgery.
A second multivariate Cox proportional hazards regression survival model was created using the dataset after propensity score-matching approach (PSM) (6). This model was constructed in the same manner as the first model. The PSM with a 1:1 ratio was performed comparing outcomes with surgery versus non-surgery using the nearest-neighbor match on the logit of the propensity score for following variables including age, gender, race, region, and grade of differentiation. The caliper width was 0.003 times the standard deviation of the logit of the propensity score. Subgroup analyses were conducted for grade of differentiation and 1-year survival after diagnosis.
Statistical tests were performed using Stata, version 13.1 (StataCorp). The assumption of proportionality for all Cox proportional hazards regression models were verified graphically using log-log survival plots. All statistical tests were 2-sided and P<0.05 was considered statistically significant.
Results
Patients’ characteristics
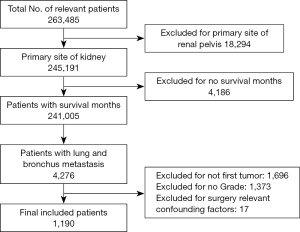
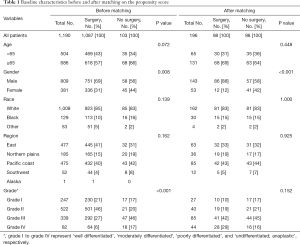
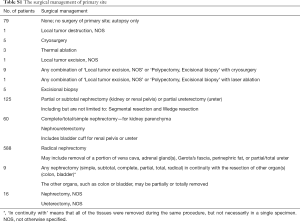
Carefully screening and analysis was performed for data extracted from the SEER database. Firstly, we extracted 263,485 relevant patients from the database. Patients with primary site of kidney, survival months and LBM were included. However, Patients with primary site of renal pelvis, no survival months and other limited data were excluded. Finally, A total of 1,190 RCC patients with LBM were included, of whom 1,087 patients underwent surgery and 103 patients unperformed surgery (Figure 1). A PSM was performed matching 98 patients receiving surgery with 98 patients unperformed surgery. Patients characteristics were well balanced across all covariates (Table 1). The surgical management of primary site was shown in Table S1.
Full table
Full table
Survival outcomes
We analyzed the correlation of surgery and survival outcome of RCC patients with LBM. The median survival time was 56 months (95% CI, 54 to 59) for the surgery group, and 6 months (95% CI, 5 to 7) for non-surgery group. K-M analysis (Figure 2A) results revealed that LBM patients underwent surgery had significantly longer survival time (log-rank test, P<0.001). We comparing outcomes with surgery versus no surgery after the PSM. As shown in Figure 2B, longer survival time was observed in LBM patients underwent surgery demonstrated by K-M analysis (log-rank test, P<0.001). In univariate analysis (Table 2), the survival of RCC patients was significantly associated with surgery (P<0.001), grade II (P=0.014), grade III (P=0.001) and grade IV (P<0.001). Moreover, multivariate analysis indicated that surgery (P<0.001), grade II (P=0.018), grade III (P<0.001) and grade IV (P<0.001) were independent prognostic indexes for OS.
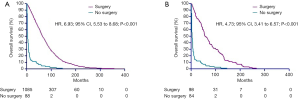
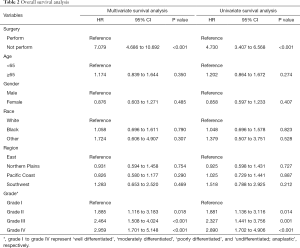
Full table
Subgroup analysis
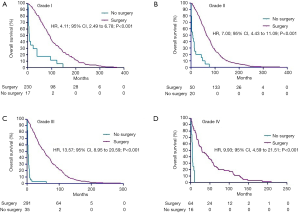
Considering the limitation especially sample size of enrolled patients, all subgroup analyses were performed before PSM. In the subgroups of survival more than 1 year after diagnosis, longer survival times were seen in the surgery arms (Figure 3, P<0.001). In addition, longer survival times were observed in CN arms in the subgroups of grade I, II, III and IV (Figure 4A,B,C,D, all P<0.001).
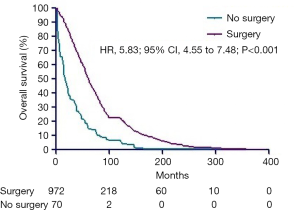
Discussion
With the improvement of diagnosis and treatment, the 5-year survival rate of RCC has reached 69.2%. But about 30% of RCC patients had distant metastasis at the time of initial diagnosis. About 30% of patients diagnosed with localized RCC who received nephrectomy would eventually emerge a distant metastasis. From the perspective of our existing clinical experience, mRCC has a poor prognosis, since the median survival time is only 6–10 months, and the 2-year median survival rate is only 10% to 20%. The site of metastasis may be related to the histological type of RCC. Study has elaborated that the proportion of lung metastasis in clear cell carcinoma, papillary cell carcinoma and chromophobe cell carcinoma is 53.6%, 33.3% and 28.2%, respectively (7).
At present, there is no consensus on the best clinical strategy to manage RCC patients with LBM. However, comprehensive treatment including nephrectomy, surgical metastasectomy, local ablation techniques, CN and molecular targeted therapy is the a suitable and commonly option for most urologists and oncologists.
The primary aim of local ablation techniques is to protect organ functions and to maintain organ integrity without compromising the oncological outcome. It was reported that local ablation techniques are ideal treatments for selected RCC patients (8-10).
CN refers to the surgical treatment of nephrectomy for mRCC patients (11). Early CN is a palliative treatment for mRCC patients to alleviate pain, hematuria, paraneoplastic syndrome, and tumor-related symptoms associated with adjacent organs (12). A large retrospective study from Japan included 164 patients with mRCC, including 133 (81.1%) with lung metastases. All patients underwent CN, followed by immunotherapy and/or targeted therapy with a median OS of 25.8 months. The authors believed that treatment with molecular-targeted agents following CN may contribute to improve the survival of patients with mRCC compared with immunotherapy alone (13).
Due to the limited sensitivity to radiotherapy, chemotherapy, endocrine therapy and immunotherapy, surgical metastasectomy of LBM was the only effective treatment prior to the advent of molecular targeted therapy in mRCC (14-17). However, the deficiency of large-sample-sized research evidence lead to the difficulties in definition and selection of indications in metastasectomy. Studies have yielded that the 5-year survival rate after metastases resection in mRCC patients with single lung metastases ranged from 21% to 83% (18,19). Additionally, a large retrospective study demonstrated that RCC patients who had lung-only metastases had a higher 5-year cancer-specific survival rate with complete metastasectomy in comparison to incomplete metastasectomy (73.6% vs. 19%, P<0.001) (20). In terms of the comparison between surgical and non-surgical treatments, Dabestani and his colleagues have reported that the median survival of patients with lung metastases resection compared with molecular targeted therapy and immunotherapy is significantly improved (36.3 vs. 30.4 and 18.0 months, P<0.001) (21). In our study, surgery might bring significant survival benefits to RCC patients with LBM whether in the overall analysis or in the subgroup analyses, which was consistent with the above results. However, the limitation in patients of no-surgery arm could expand the bias in analysis. A remarkable fact is that the details of surgical intervention were not specifically described in our study. Considering the limitation of patients after stratification analysis, we did not stratify RCC patients with LBM according to the surgical approach in the SEER database.
A large number of multi-center clinical retrospective studies have confirmed that, in general, surgical intervention for RCC patients with LBM can significantly improve prognosis. In addition to considering prognostic improvements, the economic factor of patients also needs to be considered. The cost of surgery is much lower than that of molecular targeted therapy, especially in developing countries (22,23). Due to the high cost of molecular targeted therapy, surgery might be the only choice for a part of RCC patients with LBM, to whom the maximal resection of tumor lesions is essential. Surgery combined with molecular targeted therapy is the best choice for patients in selected. Besides, surgery monotherapy is also a reasonable choice for patients with economic burden.
Noteworthily, limitations were existed in our study. Firstly, no clinical samples but only data from SEER database was enrolled. Secondly, data sources were based on SEER database. Because we were unable to fully access the patients’ information in other databases such as NCDB database and NPCR database (24,25), the data source was only extracted from SEER database in our study, which increased the bias, reduced the completeness and reliability of results.
Conclusions
Comparing to non-surgical options, RCC patients with LBM who underwent surgery might have a significantly longer survival time. In consequence, surgery should be the preferred choice for eligible RCC patients with LBM.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 81702520).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Rueckert J, Devitt K, Gardner JA. Renal Cell Carcinoma with monosomy 8: A Case Series and Review of the Literature. J Assoc Genet Technol 2018;44:5-9. [PubMed]
- Chandrasekar T, Klaassen Z, Goldberg H, et al. Metastatic renal cell carcinoma: Patterns and predictors of metastases-A contemporary population-based series. Urol Oncol 2017;35:661.e7-14. [Crossref] [PubMed]
- de Velasco G, Wankowicz SA, Madison R, et al. Targeted genomic landscape of metastases compared to primary tumours in clear cell metastatic renal cell carcinoma. Br J Cancer 2018;118:1238-42. [Crossref] [PubMed]
- Available online: https://seer.cancer.gov/data-software/documentation/seerstat/nov2017/, 2018-08-20.
- Peikes DN, Moreno L, Orzol SM. Propensity Score Matching. American Statistician 2008;62:222-31. [Crossref]
- Roncati L, Maiorana A. Biological characterization of metastatic renal cell carcinoma. Urologia 2010;77 Suppl 16:37-41. [Crossref] [PubMed]
- Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng 2005;33:223-31. [Crossref] [PubMed]
- Di Candio G, Porcelli F, Campatelli A, et al. High-Intensity Focused Ultrasonography and Radiofrequency Ablation of Renal Cell Carcinoma Arisen in Transplanted Kidneys: Single-Center Experience With Long-Term Follow-Up and Review of Literature. J Ultrasound Med 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Samadi K, Arellano RS. Ureteral protection during microwave ablation of renal cell carcinoma: combined use of pyeloperfusion and hydrodissection. Diagn Interv Radiol 2018;24:388-91. [Crossref] [PubMed]
- Joshi SS, Handorf EA, Zibelman M, et al. Treatment Facility Volume and Survival in Patients with Metastatic Renal Cell Carcinoma: A Registry-based Analysis. Eur Urol 2018;74:387-93. [Crossref] [PubMed]
- Bex A, Haanen J. Cytoreductive nephrectomy in metastatic renal cancer - less is more. Nat Rev Clin Oncol 2018;15:595-6. [Crossref] [PubMed]
- Sakai I, Miyake H, Hinata N, et al. Improved survival in patients with metastatic renal cell carcinoma undergoing cytoreductive nephrectomy in the era of targeted therapy. Int J Clin Oncol 2014;19:674-8. [Crossref] [PubMed]
- De Wolf K, Vermaelen K, De Meerleer G, et al. The potential of radiotherapy to enhance the efficacy of renal cell carcinoma therapy. Oncoimmunology 2015;4:e1042198. [Crossref] [PubMed]
- Jonasch E, Gao J, Rathmell WK. Renal cell carcinoma. BMJ 2014;349:g4797. [Crossref] [PubMed]
- Pal SK, Haas NB. Adjuvant therapy for renal cell carcinoma: past, present, and future. Oncologist 2014;19:851-9. [Crossref] [PubMed]
- Sun M, Meyer CP, Karam JA, et al. Predictors, utilization patterns, and overall survival of patients undergoing metastasectomy for metastatic renal cell carcinoma in the era of targeted therapy. Eur J Surg Oncol 2018;44:1439-45. [Crossref] [PubMed]
- Tsakiridis K, Visouli AN, Zarogoulidis P, et al. Lost in time pulmonary metastases of renal cell carcinoma: complete surgical resection of metachronous metastases, 18 and 15 years after nephrectomy. J Thorac Dis 2012;4 Suppl 1:69-73. [PubMed]
- Hofmann HS, Neef H, Krohe K, et al. Prognostic factors and survival after pulmonary resection of metastatic renal cell carcinoma. Eur Urol 2005;48:77-81; discussion 81-2. [Crossref] [PubMed]
- Alt AL, Boorjian SA, Lohse CM, et al. Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer 2011;117:2873-82. [Crossref] [PubMed]
- Dabestani S, Marconi L, Hofmann F, et al. Local treatments for metastases of renal cell carcinoma: a systematic review. Lancet Oncol 2014;15:e549-61. [Crossref] [PubMed]
- Flotte TR. Ethical Implications of the Cost of Molecularly Targeted Therapies. Hum Gene Ther 2015;26:573-4. [Crossref] [PubMed]
- Borovicka JH, Calahan C, Gandhi M, et al. Economic burden of dermatologic adverse events induced by molecularly targeted cancer agents. Arch Dermatol 2011;147:1403-9. [Crossref] [PubMed]
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol 2017;3:1722-8. [Crossref] [PubMed]
- Announcement: 25th Anniversary of National Program of Cancer Registries, 1992-2017. MMWR Morb Mortal Wkly Rep 2017;66:92. [Crossref] [PubMed]