
Malignant pleural effusion and cancer of unknown primary site: a review of literature
Introduction
Metastatic cancer of unknown primary (CUP) site refers to a heterogeneous group of cancers that are detected histologically at metastatic site but with unknown primary location despite a thorough and extensive medical evaluation (1,2). They account for 4–5% of all invasive cancers. More than half of these patients will be identified to have adenocarcinoma with multiple sites of metastasis (3). Isolated pleural effusion, as the initial presenting site for CUP is uncommon. Most of these patients portend a poor prognosis (3).
A malignant pleural effusion (MPE) is defined as the presence of malignant cells in pleural fluid as noted in cytology or histology of pleural tissue biopsy obtained during thoracotomy, thoracoscopy or autopsy (4). MPE is a very common complication of advanced malignancies and is the most important diagnosis to exclude in unilateral pleural effusions. The leading etiology of MPE in both sexes is lung cancer (35.6%), lymphoma/leukemia (15.9%) and breast cancer (14.8%) (5). In one study evaluating 5888 MPE specimens, the incidence of cancer with unknown primary was 10.2% (5).
Despite all the recent advances in Oncology, the diagnosis and treatment of MPE associated with CUP remains a dilemma for physicians with no clear guidelines or consensus.
Discussion
MPE
The most common cause of MPE tends to be lung cancers. In one analysis of 5,888 MPE specimens, 35.6% were of MPE were noted to be from the lung (5). Together, 50–65% of MPE are either from lung or breast cancer (6). Clinically, dyspnea and cough are the most common presenting features of MPE. The degree of dyspnea has a positive correlation with size of pleural effusion and a therapeutic thoracentesis usually results in relief of dyspnea (7). Some patients may also have vague constitutional symptoms, however none of these symptoms elude to the localization of the primary tumor. Of note, up to 25% of patients with MPE are asymptomatic at the time of diagnosis (8). On chest imaging (Figure 1), a majority of MPE present as ipsilateral effusions although 10–13% can be bilateral (9,10). The 3/4th of MPE are moderate to large in size, with volumes ranging between 0.5–2 L (5). These effusions can cause complete opacification of the hemithorax and are termed as a massive pleural effusion. When present, they can be a strong indication of malignancy (11).
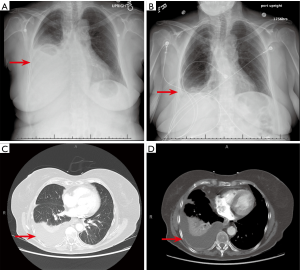
Paraneoplastic effusions (also known as paramalignant effusions) are effusions seen in tumors without direct pleural involvement and no evidence of malignant cells in pleural fluid. These effusions are usually caused by conditions that coexist with tumors, such as pulmonary embolism, thoracic duct obstruction, compression of the superior vena cava, pericardial infiltration, hypoalbuminemia, obstructive pneumonia, or atelectasis (4). Paraneoplastic pleural effusions tend to have a better prognosis than MPE (12). Differentiating between MPE and paraneoplastic effusions is important and always requires a thoracentesis with pleural fluid analysis. MPEs are mostly exudative with a protein concentration of about 4 mg/dL (8). Bloody malignant effusion can result from tumor induced angiogenesis, direct vascular invasion of tumor, venule occlusion and increased capillary permeability by release of vasoactive factors (13). The red cell count of these effusions are usually in the range 30,000–50,000 per microliter (5). The lymphocytes are usually numerous and in the range of 50–70% with a predominance of T cells (5). One third of MPEs have a low pleural fluid pH (<7.3) and pleural glucose concentration (<60 mg/dL) (14,15). Low pleural fluid pH and glucose concentration in MPE is associated with poorer prognosis. They are also indicative of extensive disease and lower chance of successful pleurodesis (14). In one study, shorter survival was noted with pleural fluid pH <7.28 [median survival of 2.5 months and a 3-month survival of 38.9% (95% CI: 31.1% to 46.8%)] versus pH >7.28 [median survival of 4.3 months and 3-month survival of 61.6% (95% CI: 55.7% to 67.4%)] (16).
Pleural biopsy might be necessary if the pleural fluid analysis was insufficient to make a diagnosis or if the tissue yield is inadequate for immunohistochemical staining. The biopsy can be done by several methods including blind-closed percutaneous needle biopsies and image guided needle biopsies. More recently the popularity of local anesthetic thoracoscopy (LAT) (aka medical thoracoscopy) (17,18), or video-assisted thoracoscopic surgery (VATS) has been increasing given their safety profile. When dealing with malignancy, image guided biopsy or thoracoscopy (medical or surgical) are very reliable in obtaining sufficient tissue to facilitate diagnosis (4).
CUP
Metastatic CUP site account for 4–5% of all invasive cancers. More than half of these tumors identified to be adenocarcinoma with multiple sites of metastasis (3). CUP usually lack early clinical symptoms due to the fact of absence of primary tumor which may explain the aggressive behavior seen with them, such as early dissemination and unpredictability of metastatic pattern. Extensive medical evaluation for CUP should include, a detailed medical history, complete physical examination, full blood count and biochemistry, urinalysis and stool occult blood testing, histopathological review of biopsy material with the use of immunohistochemistry, chest radiography, and CT of the abdomen and pelvis (2). In one study, ~11% of the MPE were deemed to be from CUP despite an extensive work up (5). Unfortunately, the diagnostic yield after a single thoracentesis for malignancy from pleural fluid samples is just 60% (19). CUP typically tend to be poorly differentiated cancers and may not demonstrate typical immunocytochemistry (IHC) stains with clonal evolution of cell lineage. When seen with MPE there is often a need for pleural biopsies to confirm or exclude a primary tumor diagnosis. Finally, while it is important to state the importance of using gene expression profiling to guide suspected site-specific treatment of CUP; site specific treatment based on microarray profiling does not improve 1-year survival (16.7 vs. 10.6 months; P=0.116) compared to empiric paclitaxel and carboplatin (20). The use of an established algorithm to predict the primary site of CUP does have prognostic value (20).
Hybrid positron emission tomography-computed tomography (PET-CT) should be utilized in the workup of CUP since it aids in the identification of primary site and hence affect management algorithm (21). In a 2006 study, 68 consecutive patients with CUP syndrome underwent whole body FDG-PET/CT as part of diagnostic workup. In this study, the primary tumor site was identified in 35.3% of patients (22). Unexpected metastases were identified in ~13% of these patients resulting in restaging. Overall, the final oncological treatment was influenced by PET-CT in 48.5% of these patients (22). A 2009 systematic review of 11 studies (433 patients with CUP) examined the use of combined FDG-PET/CT in the detection of unknown primary tumors. The overall primary tumor detection rate in this paper was 37% (23); 4/11 studies in this review showed that PET-CT modified therapy in 18.2–60% of patients (23). In a more recent 2016 recent study involving 82 patients with CUP syndrome, PET-CT scan identified the primary tumor site in 57.3% of cases and upstaged the disease in 27% of cases (24). Overall, PET-CT in CUP was found to have a diagnostic accuracy of 78%, sensitivity of 80%, specificity of 74%, positive predictive value of 88.7% and negative predictive value of 59% (24). While there is substantial data to support the use of FDG-PET/CT in the detection of primary tumor sites, but one must keep in mind that this imaging modality is expensive and not always available in some regions. Furthermore, false positive results can lead to unnecessary secondary diagnostic procedures with associated risk of complications.
Recent advances in management of MPE and CUP
The use of immunocytochemistry (IHC) studies and biomarkers of MPE has proven useful in helping to evaluate the primary tumor histology in patients with CUP (25,26). IHC of a CUP biopsy includes a three-step approach:
- Diagnose the broad type of cancer (carcinoma, sarcoma, melanoma or lymphoma);
- Detect the subtypes (adenocarcinoma, germ-cell tumor, hepatocellular, renal, thyroid, neuroendocrine, or squamous carcinoma);
- Provide information about primary site of cancer (prostate, lung, breast, colon, pancreas or biliary, or ovarian cancer).
Pomjanski et al. evaluated the malignant effusions (118 pleural, 53, peritoneal, and 9 pericardial) of 180 CUP patients for the presence of 6 monoclonal antibodies: Cytokeratin (CK) 5/6, CK 7, CK 20, cancer antigen (CA) 125, and thyroid transcription factor (TTF)-1. An algorithmic approach was employed utilizing these 6 antibodies to identify the primary tumor site. This approach correctly identified 85% of the primary tumors (94% ovaries and 88% lungs) (27).
The identification of specific biomarkers for malignancy, especially in lung cancer, is vital for not only improving the accuracy of diagnosis but also guiding therapy. Evaluating for markers like epidermal growth factor (EGFR), v-Raf murine sarcoma viral oncogene homolog B (BRAF), Kirsten rat sarcoma virus (KRAS), and for translocations in gene rearrangement anaplastic lymphoma kinase (ALK), rat osteosarcoma (ROS1) can predict tumor behavior and assess patient candidacy for gene targeted therapy (26). Also, the presence of one of these markers may help suggest that the MPE in the CUP is of lung origin (28). It has also been shown that pleural fluid oncogenic marker detection correlates well with the markers noted in tissue samples from the primary tumor in lung cancer (29). With the recent advancement of cancer therapy and the introduction of monoclonal antibodies including immune checkpoint inhibitors (ICIs), identification of these targetable oncogenic mutation has gained limelight in research. There have been few reported cases (Table 1) of disease control being achieved in patients with CUP treated with monoclonal antibodies that mainly targeted EGFR, HER2 and VEGF antigens (31-33). Similarly, Nivolumab and Pembrolizumab are two immune checkpoint inhibitors (ICI) that are approved by FDA targeting PD-1, while Atezolizumab, Durvalumab and Avelumab are three ICI targeting PD-L1. Scarce data available on the use of ICI to treat patient with CUP. However, there are cases were reported in literature (Table 1) with varying degree of response (34-36). An evolving area is the use of agents such as VEGF inhibitors (Bevacizumab), to improve the pleural permeability of conventional chemotherapeutic agents (37). This could allow for higher bioavailability of the drugs at the site of tumor at potentially lower doses.
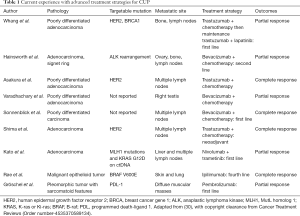
Full table
Another emerging science in the field of Oncology is liquid biopsy (38). It refers to the development of technology to identify circulating tumor cell free DNA (cfDNA) in the blood. The hypothesis of being able to detect these mutations in blood for patients with CUP is intriguing since these abnormalities will generate from both the metastatic and primary tumor site (39). In one study involving 442 patients with CUP, 80% of the patients had cfDNA alterations detected in the blood and of those 87% had distinct genetic profiles of which 99% had targetable mutations (34). Although interesting, there may not be enough evidence for liquid biopsy to routinely be utilized in patients with CUP (39).
The prognosis of patients with CUP (with MPE) remains poor despite the recent advancement in therapy. The mean survival of these patient (untreated) is estimated to be 4–6 months, with one-year survival rate of around 18% associated with significant symptoms, poor quality of life, and extensive utilization of medical resources (1,2,4). As the focus of therapy should be to alleviate symptoms and improve quality of life, indwelling pleural catheters (IPC) or palliative pleurodesis should be considered in the setting of recurrent symptomatic pleural effusions under controlled with optimal tumor therapy. It is well known that IPC utilization leads to decreased hospital stay, improved quality of life, and reduction of dyspnea while maintaining good safety profile with low complications in long term use (40). Other measure considered included decortication and pleuroperitoneal shunts (41). For patients with very short life expectancy, serial thoracentesis is a reasonable option for dyspnea relief (41).
Conclusions
In this paper, we provided an overview of MPE from CUP including diagnosis, prognosis and treatment options. About 11% of MPE are due to CUP. As with all cases of CUP, the first step should be complete an extensive medical evaluation with appropriate diagnostic testing. A thoracentesis is vital diagnostic step and is required to differentiate neoplastic from paraneoplastic effusion. Additional methods for tissue sampling should be considered if the initial pathology is non-revealing or if the tissue yield is poor. Immunohistochemical testing of fluid cytology and pleural biopsy samples can elude to the primary malignancy and guide therapy, as most of these cases are felt to be from lung cancer. With the advent of gene directed therapies and immune checkpoint inhibitors, there are new therapies being considered for these patients. Overall, MPEs from a CUP has a poor prognosis and symptom alleviating measures should be part of the management plan.
Acknowledgments
None.
Footnote
Conflicts of Interest: MR Bowling is a consultant for Medtronic, Biodesix and Veracyte. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Briasoulis E, Tolis C, Bergh J, et al. ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of cancers of unknown primary site (CUP). Ann Oncol 2005;16 Suppl 1:i75-6. [Crossref] [PubMed]
- Pavlidis N, Fizazi K. Carcinoma of unknown primary (CUP). Crit Rev Oncol Hematol 2009;69:271-8. [Crossref] [PubMed]
- Briasoulis E, Pavlidis N. Cancer of Unknown Primary Origin. Oncologist 1997;2:142-52. [PubMed]
- Sahn SA. Pleural diseases related to metastatic malignancies. Eur Respir J 1997;10:1907-13. [Crossref] [PubMed]
- Johnston WW. The malignant pleural effusion. A review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer 1985;56:905-9. [Crossref] [PubMed]
- Roberts ME, Neville E, Berrisford RG, et al. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii32-40. [Crossref] [PubMed]
- Dixit R, Agarwal KC, Gokhroo A, et al. Diagnosis and management options in malignant pleural effusions. Lung India 2017;34:160-6. [Crossref] [PubMed]
- Chernow B, Sahn SA. Carcinomatous involvement of the pleura: an analysis of 96 patients. Am J Med 1977;63:695-702. [Crossref] [PubMed]
- Banerjee AK, Willetts I, Robertson JF, et al. Pleural effusion in breast cancer: a review of the Nottingham experience. Eur J Surg Oncol 1994;20:33-6. [PubMed]
- Laisaar T, Palmiste V, Vooder T, et al. Life expectancy of patients with malignant pleural effusion treated with video-assisted thoracoscopic talc pleurodesis. Interact Cardiovasc Thorac Surg 2006;5:307-10. [Crossref] [PubMed]
- Maher GG, Berger HW. Massive pleural effusion: malignant and nonmalignant causes in 46 patients. Am Rev Respir Dis 1972;105:458-60. [PubMed]
- Ferreiro L, Suarez-Antelo J, Valdes L. Pleural procedures in the management of malignant effusions. Ann Thorac Med 2017;12:3-10. [Crossref] [PubMed]
- Meyer PC. Metastatic carcinoma of the pleura. Thorax 1966;21:437-43. [Crossref] [PubMed]
- Sahn SA, Good JT Jr. Pleural fluid pH in malignant effusions. Diagnostic, prognostic, and therapeutic implications. Ann Intern Med 1988;108:345-9. [Crossref] [PubMed]
- Good JT Jr, Taryle DA, Sahn SA. The pathogenesis of low glucose, low pH malignant effusions. Am Rev Respir Dis 1985;131:737-41. [PubMed]
- Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of survival for patients with malignant pleural effusions. Chest 2000;117:79-86. [Crossref] [PubMed]
- Murthy V, Bessich JL. Medical thoracoscopy and its evolving role in the diagnosis and treatment of pleural disease. J Thorac Dis 2017;9:S1011-S21. [Crossref] [PubMed]
- Wu YB, Xu LL, Wang XJ, et al. Diagnostic value of medical thoracoscopy in malignant pleural effusion. BMC Pulm Med 2017;17:109. [Crossref] [PubMed]
- Brock MV, Hooker CM, Yung R, et al. Can we improve the cytologic examination of malignant pleural effusions using molecular analysis? Ann Thorac Surg 2005;80:1241-7. [Crossref] [PubMed]
- Hayashi H, Kurata T, Takiguchi Y, et al. Randomized Phase II Trial Comparing Site-Specific Treatment Based on Gene Expression Profiling With Carboplatin and Paclitaxel for Patients With Cancer of Unknown Primary Site. J Clin Oncol 2019;37:570-9. [Crossref] [PubMed]
- Reske SN, Kotzerke J. FDG-PET for clinical use. Results of the 3rd German Interdisciplinary Consensus Conference, "Onko-PET III", 21 July and 19 September 2000. Eur J Nucl Med 2001;28:1707-23. [Crossref] [PubMed]
- Pelosi E, Pennone M, Deandreis D, et al. Role of whole body positron emission tomography/computed tomography scan with 18F-fluorodeoxyglucose in patients with biopsy proven tumor metastases from unknown primary site. Q J Nucl Med Mol Imaging 2006;50:15-22. [PubMed]
- Kwee TC, Kwee RM. Combined FDG-PET/CT for the detection of unknown primary tumors: systematic review and meta-analysis. Eur Radiol 2009;19:731-44. [Crossref] [PubMed]
- Riaz S, Nawaz MK, Faruqui ZS, et al. Diagnostic Accuracy of 18F-Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography in the Evaluation of Carcinoma of Unknown Primary. Mol Imaging Radionucl Ther 2016;25:11-8. [Crossref] [PubMed]
- Afify AM, al-Khafaji BM. Diagnostic utility of thyroid transcription factor-1 expression in adenocarcinomas presenting in serous fluids. Acta Cytol 2002;46:675-8. [Crossref] [PubMed]
- Porcel JM. Biomarkers in the diagnosis of pleural diseases: a 2018 update. Ther Adv Respir Dis 2018;12:1753466618808660. [Crossref] [PubMed]
- Pomjanski N, Grote HJ, Doganay P, et al. Immunocytochemical identification of carcinomas of unknown primary in serous effusions. Diagn Cytopathol 2005;33:309-15. [Crossref] [PubMed]
- Liu N, Sun RZ, Du J, et al. Comparison of Epidermal Growth Factor Receptor Gene Mutations Identified Using Pleural Effusion and Primary Tumor Tissue Samples in Non-Small Cell Lung Cancer. Appl Immunohistochem Mol Morphol 2018;26:e44-51. [PubMed]
- DeMaio A, Clarke JM, Dash R, et al. Yield of Malignant Pleural Effusion for Detection of Oncogenic Driver Mutations in Lung Adenocarcinoma. J Bronchology Interv Pulmonol 2019;26:96-101. [Crossref] [PubMed]
- El Rassy E, Pavlidis N. The current evidence for a biomarker-based approach in cancer of unknown primary. Cancer Treat Rev 2018;67:21-8. [Crossref] [PubMed]
- Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med 2007;357:39-51. [Crossref] [PubMed]
- Schliemann C, Neri D. Antibody-based vascular tumor targeting. Recent Results Cancer Res 2010;180:201-16. [Crossref] [PubMed]
- Asakura H, Takashima H, Mitani M, et al. Unknown primary carcinoma, diagnosed as inflammatory breast cancer,and successfully treated with trastuzumab and vinorelbine. Int J Clin Oncol 2005;10:285-8. [Crossref] [PubMed]
- Kato S, Krishnamurthy N, Banks KC, et al. Utility of Genomic Analysis In Circulating Tumor DNA from Patients with Carcinoma of Unknown Primary. Cancer Res 2017;77:4238-46. [Crossref] [PubMed]
- Groschel S, Bommer M, Hutter B, et al. Integration of genomics and histology revises diagnosis and enables effective therapy of refractory cancer of unknown primary with PDL1 amplification. Cold Spring Harb Mol Case Stud 2016;2:a001180. [Crossref] [PubMed]
- Roe OD, Wahl SG. The undifferentiated carcinoma that became a melanoma: Re-biopsy of a cancer of an unknown primary site: a case report. J Med Case Rep 2017;11:82. [Crossref] [PubMed]
- Stathopoulos GT, Kalomenidis I. Malignant pleural effusion: tumor-host interactions unleashed. Am J Respir Crit Care Med 2012;186:487-92. [Crossref] [PubMed]
- Palmirotta R, Lovero D, Cafforio P, et al. Liquid biopsy of cancer: a multimodal diagnostic tool in clinical oncology. Ther Adv Med Oncol 2018;10:1758835918794630. [Crossref] [PubMed]
- El Rassy E, Khaled H, Pavlidis N. Liquid biopsy: a new diagnostic, predictive and prognostic window in cancers of unknown primary. Eur J Cancer 2018;105:28-32. [Crossref] [PubMed]
- Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of Malignant Pleural Effusions. An Official ATS/STS/STR Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:839-49. [Crossref] [PubMed]
- Aydin Y, Turkyilmaz A, Intepe YS, et al. Malignant pleural effusions: appropriate treatment approaches. Eurasian J Med 2009;41:186-93. [PubMed]


